Abstract
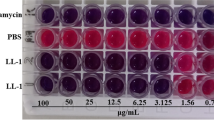
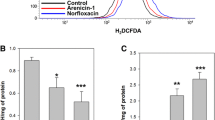
Helveticin-M, a novel Class III bacteriocin produced by Lactobacillus crispatus exhibited an antimicrobial activity against Staphylococcus aureus, S. saprophyticus, and Enterobacter cloacae. To understand how Helveticin-M injured target cells, Helveticin-M was cloned and heterologously expressed in Escherichia coli. Subsequently, the cell wall organization and cell membrane integrity of target cells were determined. The mechanism of cellular damage differed according to bacterial species. Based on morphology analysis, Helveticin-M disrupted the cell wall of Gram-positive bacteria and disorganized the outer membrane of Gram-negative bacteria, therefore, altering surface structure. Helveticin-M also disrupted the inner membrane, as confirmed by leakage of intracellular ATP from cells and depolarization of membrane potential of target bacteria. Based on cell population analysis, Helveticin-M treatment caused the increase of cell membrane permeability, but the cytosolic enzymes were not influenced, indicating that it was the sublethal injury. Therefore, the mode of Helveticin-M action is bacteriostatic rather than bactericidal.









Similar content being viewed by others
References
Agcam E, Akyildiz A, Akdemir EG (2014) Comparison of phenolic compounds of orange juice processed by pulsed electric fields (PEF) and conventional thermal pasteurisation. Food Chem 143:354–361. https://doi.org/10.1016/j.foodchem.2013.07.115
Baneyx F, Mujacic M (2004) Recombinant protein folding and misfolding in Escherichia coli. Nat Biotechnol 22:1399–1408
Chen B, Fan DQ, Zhu KX, Shan ZG, Chen FY, Hou L, Cai L, Wang KJ (2015) Mechanism study on a new antimicrobial peptide Sphistin derived from the N-terminus of crab histone H2A identified in haemolymphs of Scylla paramamosain. Fish Shellfish Immunol 47:833–846. https://doi.org/10.1016/j.fsi.2015.10.010
Chopra L, Singh G, Kumar JK, Sahoo DK (2015) Sonorensin: a new bacteriocin with potential of an anti-biofilm agent and a food biopreservative. Sci Rep 5:13412. https://doi.org/10.1038/srep13412
Cleveland J, Montville TJ, Nes IF, Chikindas ML (2001) Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol 71:1–20
Cotter PD, Ross RP, Hill C (2013) Bacteriocins—a viable alternative to antibiotics? Nat Rev Microbiol 11:95–105. https://doi.org/10.1038/nrmicro2937
Eijsink VG, Axelsson L, Diep DB, Havarstein LS, Holo H, Nes IF (2002) Production of class II bacteriocins by lactic acid bacteria; an example of biological warfare and communication. Antonie Van Leeuwenhoek 81:639–654
Gharsallaoui A, Oulahal N, Joly C, Degraeve P (2016) Nisin as a food preservative: part 1: physicochemical properties, antimicrobial activity, and main uses. Crit Rev Food Sci Nutr 56:1262–1274. https://doi.org/10.1080/10408398.2013.763765
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp 41:95–98
Hoffmann M, Luo Y, Monday SR, Gonzalez-Escalona N, Ottesen AR, Muruvanda T, Wang C, Kastanis G, Keys C, Janies D, Senturk IF, Catalyurek UV, Wang H, Hammack TS, Wolfgang WJ, Schoonmaker-Bopp D, Chu A, Myers R, Haendiges J, Evans PS, Meng J, Strain EA, Allard MW, Brown EW (2016) Tracing Origins of the Salmonella bareilly strain causing a food-borne outbreak in the United States. J Infect Dis 213:502–508. https://doi.org/10.1093/infdis/jiv297
Hultman J, Rahkila R, Ali J, Rousu J, Bjorkroth KJ (2015) Meat processing plant microbiome and contamination patterns of cold-tolerant bacteria causing food safety and spoilage risks in the manufacture of vacuum-packaged cooked sausages. Appl Environ Microbiol 81:7088–7097. https://doi.org/10.1128/AEM.02228-15
Hurdle JG, O’Neill AJ, Chopra I, Lee RE (2010) Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat Rev Microbiol 9:62
Joerger MC, Klaenhammer TR (1990) Cloning, expression, and nucleotide sequence of the Lactobacillus helveticus 481 gene encoding the bacteriocin helveticin. J Bacteriol 172:6339–6347
Kadariya J, Smith TC, Thapaliya D (2014) Staphylococcus aureus and staphylococcal food-borne disease: an ongoing challenge in public health. Biomed Res Int 2014:827965. https://doi.org/10.1155/2014/827965
Lavieri NA, Sebranek JG, Cordray JC, Dickson JS, Horsch AM, Jung S, Manu DK, Mendonça AF, Stecher BB (2015) Control of Listeria monocytogenes on alternatively cured ready-to-eat ham using natural antimicrobial ingredients in combination with post-lethality interventions. Food Process Technol 6:10
Lazdunski CJ (1995) Colicin import and pore formation: a system for studying protein transport across membranes? Mol Microbiol 16:1059–1066
Lee H, Kim HY (2011) Lantibiotics, class I bacteriocins from the genus Bacillus. J Microbiol Biotechnol 21:229–235
Li J, Ahn J, Liu D, Chen S, Ye X, Ding T (2016) Evaluation of ultrasound-induced damage to Escherichia coli and Staphylococcus aureus by flow cytometry and transmission electron microscopy. Appl Environ Microbiol 82:1828–1837. https://doi.org/10.1128/AEM.03080-15
Liu G, Song Z, Yang X, Gao Y, Wang C, Sun B (2016) Antibacterial mechanism of bifidocin A, a novel broad-spectrum bacteriocin produced by Bifidobacterium animalis BB04. Food Control 62:309–316
Liu YG, Chen Y (2007) High-efficiency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences. Biotechniques 43:649–650, 652, 654
Lou M, Zhu B, Muhammad I, Li B, Xie G, Wang Y, Li H, Sun G (2011) Antibacterial activity and mechanism of action of chitosan solutions against apricot fruit rot pathogen Burkholderia seminalis. Carbohyd Res 346:1294–1301. https://doi.org/10.1016/j.carres.2011.04.042
Lou MM, Zhu B, Muhammad I, Li B, Xie GL, Wang YL, Li HY, Sun GC (2011) Antibacterial activity and mechanism of action of chitosan solutions against apricot fruit rot pathogen Burkholderia seminalis. Carbohydr Res 346:1294–1301. https://doi.org/10.1016/j.carres.2011.04.042
Mercer RG, Walker BD, Yang X, McMullen LM, Ganzle MG (2017) The locus of heat resistance (LHR) mediates heat resistance in Salmonella enterica, Escherichia coli and Enterobacter cloacae. Food Microbiol 64:96–103. https://doi.org/10.1016/j.fm.2016.12.018
Moll GN, Konings WN, Driessen AJ (1999) Bacteriocins: mechanism of membrane insertion and pore formation. Antonie Van Leeuwenhoek 76:185–198
NicAogain K, O’Byrne CP (2016) The role of stress and stress adaptations in determining the fate of the bacterial pathogen Listeria monocytogenes in the food chain. Front Microbiol 7:1865. https://doi.org/10.3389/fmicb.2016.01865
Nigutova K, Serencova L, Piknova M, Javorsky P, Pristas P (2008) Heterologous expression of functionally active enterolysin A, class III bacteriocin from Enterococcus faecalis, in Escherichia coli. Protein Expr Purif 60:20–24. https://doi.org/10.1016/j.pep.2008.03.006
Nilsen T, Nes IF, Holo H (2003) Enterolysin A, a cell wall-degrading bacteriocin from Enterococcus faecalis LMG 2333. Appl Environ Microbiol 69:2975–2984
Oppegard C, Rogne P, Emanuelsen L, Kristiansen PE, Fimland G, Nissen-Meyer J (2007) The two-peptide class II bacteriocins: structure, production, and mode of action. J Mol Microbiol Biotechnol 13:210–219. https://doi.org/10.1159/000104750
Ozogul F, Hamed I (2017) The importance of lactic acid bacteria for the prevention of bacterial growth and their biogenic amines formation: a review. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2016.1277972
Padmavathy N, Vijayaraghavan R (2008) Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. Sci Technol Adv Mater 9:35004. https://doi.org/10.1088/1468-6996/9/3/035004
Pillet F, Formosa-Dague C, Baaziz H, Dague E, Rols MP (2016) Cell wall as a target for bacteria inactivation by pulsed electric fields. Sci Rep 6:19778. https://doi.org/10.1038/srep19778
Riley MA (1993) Molecular mechanisms of colicin evolution. Mol Biol Evol 10:1380–1395
Schenk M, Raffellini S, Guerrero S, Blanco GA, Alzamora SM (2011) Inactivation of Escherichia coli, Listeria innocua and Saccharomyces cerevisiae by UV-C light: study of cell injury by flow cytometry. Lebensmittel-Wissenschaft und-Technologie 44:191–198
Sun Z, Kong J, Hu S, Kong W, Lu W, Liu W (2013) Characterization of a S-layer protein from Lactobacillus crispatus K313 and the domains responsible for binding to cell wall and adherence to collagen. Appl Microbiol Biotechnol 97:1941–1952. https://doi.org/10.1007/s00253-012-4044-x
Sung SY, Sin LT, Tee TT, Bee ST, Rahmat AR, Rahman WAWA, Tan AC, Vikhraman M (2013) Antimicrobial agents for food packaging applications. Trends Food Sci Tech 33:110–123
Hp T, Oppedijk SF, Breukink E, Martin NI (2016) New insights into Nisin’s antibacterial mechanism revealed by binding studies with synthetic lipid II analogues. Biochemistry-Us 55:232–237. https://doi.org/10.1021/acs.biochem.5b01173
Thompson JD, Gibson TJ, Higgins DG (2002) Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinform Chapter 2:2–3. https://doi.org/10.1002/0471250953.bi0203s00
Thompson JK, Collins MA, Mercer WD (1996) Characterization of a proteinaceous antimicrobial produced by Lactobacillus helveticus CNRZ450. J Appl Bacteriol 80:338–348
Tsiraki MI, Savvaidis IN (2016) The effects of citrus extract (Citrox(c)) on the naturally occurring microflora and inoculated pathogens, Bacillus cereus and Salmonella enterica, in a model food system and the traditional Greek yogurt-based salad Tzatziki. Food Microbiol 53:150–155. https://doi.org/10.1016/j.fm.2015.09.015
Vaara M (1992) Agents that increase the permeability of the outer membrane. Microbiol Rev 56:395
Woraprayote W, Malila Y, Sorapukdee S, Swetwiwathana A, Benjakul S, Visessanguan W (2016) Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Sci 120:118–132. https://doi.org/10.1016/j.meatsci.2016.04.004
Zhang M, Wang D, Geng Z, Li P, Sun Z, Xu W (2018) Effect of heat shock protein 90 against ROS-induced phospholipid oxidation. Food Chem 240:642–647. https://doi.org/10.1016/j.foodchem.2017.08.005
Zhang T, Pan Y, Li B, Ou J, Zhang J, Chen Y, Peng X, Chen L (2013) Molecular cloning and antimicrobial activity of enterolysin A and helveticin J of bacteriolysins from metagenome of Chinese traditional fermented foods. Food Control 31:499–507
Zhao W, Yang R, Zhang HQ, Zhang W, Hua X, Tang Y (2011) Quantitative and real time detection of pulsed electric field induced damage on Escherichia coli cells and sublethally injured microbial cells using flow cytometry in combination with fluorescent techniques. Food Control 22:566–573
Zou Y, Bian H, Li P, Sun Z, Sun C, Zhang M, Geng Z, Xu W, Wang D (2017) Optimization and physicochemical properties of nutritional protein isolate from pork liver with ultrasound-assisted alkaline extraction. Anim Sci J. https://doi.org/10.1111/asj.12930
Acknowledgements
This study was supported by National Natural Science Foundation of China (31601530), the Natural Science Foundation of Jiangsu Province (BK20151367), Innovation of Agricultural Science and Technology of Jiangsu Province (CX(15)1008) and China agriculture research system (CARS-41).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Sun, Z., Wang, X., Zhang, X. et al. Class III bacteriocin Helveticin-M causes sublethal damage on target cells through impairment of cell wall and membrane. J Ind Microbiol Biotechnol 45, 213–227 (2018). https://doi.org/10.1007/s10295-018-2008-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-2008-6