Abstract
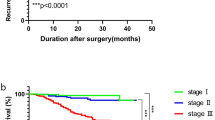
Gastrointestinal (GIT) cancers represent the third common cancers worldwide, characterized by rapid progression and higher mortality rate. Matrix metalloproteinases (MMPs) play an important role in cancer metastases. The present study was conducted to estimate and evaluate the role of MMP-7, -9, -10 and -12 and TGF β1 along with conventional biomarkers (CEA and CA19-9) in gastric (GC), pancreatic (PC) and colorectal cancer (CRC) staging system according to tumor size (T), included lymph node (N) and metastasis (M). Seventy-five patients were divided into GC group (n = 25), PC group (n = 25), CRC group (n = 25) and twenty-five healthy subjects (control group). Serum levels of MMP-7, -10 and -12 were assayed simultaneously using luminex multiplex technique. Also, MMP-9, TGF-β1, CA19-9 and CEA were determined by ELISA. MMP-7,-9,-10, -12, TGF-β1 and CEA levels were significantly (p < 0.001) higher in GIT cancer groups compared with control. CA19-9 was significantly (p < 0.001) higher in PC and CRC groups compared with control. MMP-9 was positively correlated with TNM staging in PC patients. MMP-12 was negatively correlated with T in PC and positively correlated with M in CRC group. CA 19-9 was positively correlated with M grade in CRC. Depending on the estimated cutoff values of area under receiver curve; CA19-9 and MMP-7 were excellent diagnostic markers in PC, CEA and MMP-7 were excellent in CRC, and MMP-7 and MMP-9 were excellent in GC. Our findings indicated the clinical utility of MMPs in diagnosis and TNM staging of GIT cancers along with CEA and CA19-9.



Similar content being viewed by others
Abbreviations
- GIT:
-
Gastrointestinal
- MMP:
-
Matrix metalloproteinases
- PC:
-
Pancreatic cancer
- CRC:
-
Colorectal cancer
- GC:
-
Gastric cancer
- HCC:
-
Hepatocellular carcinoma
- VEGF:
-
Vascular endothelial growth factor
- EGF:
-
Epidermal growth factor
- TGF-β1:
-
Transforming growth factor beta-1
- TIMP:
-
Tissue inhibitors of metalloproteinases
- CEA:
-
Carcinoembryonic antigen
- CA19-9:
-
Carbohydrate antigen 19-9
- ELISA:
-
Enzyme-linked immunosorbent assay
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- DSe:
-
Diagnostic sensitivity
- DSp:
-
Diagnostic specificity
- EGFR:
-
Epidermal growth factor receptor
- MEK–ERK:
-
Mitogen-activated protein kinase–extracellular signal regulated protein kinase
- TJ:
-
Tight junctions
- S100A4:
-
S100 calcium-binding protein A4
- PAI:
-
Plasminogen activator inhibitor
- HB-EGF:
-
Heparin-binding epidermal growth factor
References
Grierson P, Lim KH, Amin M. Immunotherapy in gastrointestinal cancers. J Gastrointest Oncol. 2017;8(3):474–84.
Moridikia A, Mirzaei H, Sahebkar A, Salimian J. MicroRNAs: potential candidates for diagnosis and treatment of colorectal cancer. J Cell Physiol. 2018;233(2):901–13.
Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239–48.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278(1):16–27.
Araújo RF, Lira GA, Vilaça JA, et al. Prognostic and diagnostic implications of MMP-2, MMP-9, and VEGF-α expressions in colorectal cancer. Pathol Res Pract. 2015;211(1):71–7.
Castro-Castro A, Marchesin V, Monteiro P, Lodillinsky C, Rossé C, Chavrier P. Cellular and Molecular Mechanisms of MT1-MMP-Dependent Cancer Cell Invasion. Annu Rev Cell Dev Biol. 2016;32(1):555–76.
Da Vià MC, Solimando AG, Garitano-Trojaola A, et al. CIC mutation as a molecular mechanism of acquired resistance to combined BRAF–MEK inhibition in extramedullary multiple myeloma with central nervous system involvement. Oncologist. 2020;25(2):112–8.
Zitka O, Kukacka J, Krizkova S, et al. Matrix Metalloproteinases. Biochim Biophys Acta - Mol Cell Res. 2010;1803(1):1–2.
Isaacson KJ, Martin Jensen M, Subrahmanyam NB, Ghandehari H. Matrix-metalloproteinases as targets for controlled delivery in cancer: an analysis of upregulation and expression. J Control Release. 2017;259:62–75.
Yeh Y, Sheu B. Matrix metalloproteinases and their inhibitors in the gastrointestinal cancers: current knowledge and clinical potential. Metalloproteinases In Medicine. 2014;1:3–13.
Verma S, Kesh K, Ganguly N, Jana S, Swarnakar S. Matrix metalloproteinases and gastrointestinal cancers: impacts of dietary antioxidants. World J Biol Chem. 2014;5(3):355–76.
Kahlert C, Fiala M, Musso G, et al. Prognostic impact of a compartment-specific angiogenic marker profile in patients with pancreatic cancer. Oncotarget. 2014;5(24):12978–89.
Herszényi L, Hritz I, Lakatos G, Varga MZ, Tulassay Z. The behavior of matrix metalloproteinases and their inhibitors in colorectal cancer. Int J Mol Sci. 2012;13(10):13240–63.
Klupp F, Neumann L, Kahlert C, et al. Serum MMP7, MMP10 and MMP12 level as negative prognostic markers in colon cancer patients. BMC Cancer. 2016;16:1–9.
Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–74.
Sisik A, Kaya M, Bas G, Basak F, Alimoglu O. CEA and CA 19-9 are Still Valuable Markers for the Prognosis of Colorectal and Gastric Cancer Patients. Asian Pacific J Cancer Prev. 2013;14(7):4289–94.
Kim JE, Lee KT, Lee JK, Paik SW, Rhee JC, Choi KW. Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J Gastroenterol Hepatol. 2004;19(2):182–6.
Gui JC, Yan WL, Liu XD. CA19-9 and CA242 as tumor markers for the diagnosis of pancreatic cancer: a meta-analysis. Clin Exp Med. 2014;14(2):225–33.
Amin MB, Edge S, Greene F, et al. AJCC cancer staging manual. American Joint Commission on Cancer. 8th ed. New YorkNew York: Springer; 2017.
Brierley J, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumors. 8th ed. Chichester: Wiley; 2017.
Lilliefors HW. On the Kolmogorov-Smirnov test for normality with mean and variance unknown. J Am Stat Assoc. 1967;62(318):399–402.
McKight PE, Najab J. Kruskal–Wallis test corsini encyclopedia of psychology. New York: Wiley; 2010.
Tsiaousidou A, Tsaroucha AK, Lambropoulou M, et al. Increased B7H4 tissue expression correlates with high CA19.9 serum levels and a worse prognosis of pancreatic adenocarcinoma. Clin Exp Med. 2016;16(3):351–6.
Jakubowska K, Pryczynicz A, Januszewska J, et al. Expressions of Matrix Metalloproteinases 2, 7, and 9 in Carcinogenesis of Pancreatic Ductal Adenocarcinoma. Dis Markers. 2016;2016:9895721.
Jabłońska-Trypuć A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem. 2016;31(1):177–83.
Park HD, Kang ES, Kim JW, Lee KT, Lee KH, Park YS, et al. Serum CA19-9, cathepsin D, and matrix metalloproteinase-7 as a diagnostic panel for pancreatic ductal adenocarcinoma. Proteomics. 2012;12(23–24):3590–7.
Tan X, Egami H, Abe M, Nozawa F, Hirota M, Ogawa M. Involvement of MMP-7 in invasion of pancreatic cancer cells through activation of the EGFR mediated MEK–ERK signal transduction pathway. J Clin Pathol. 2005;58(12):1242–8.
Cheng P, Jiang FH, Zhao LM, et al. Human Macrophage Metalloelastase Correlates with Angiogenesis and Prognosis of Gastric Carcinoma. Dig Dis Sci. 2010;55(11):3138–46.
Xiang T, Xia X, Yan W. Expression of Matrix Metalloproteinases-2/-9 is Associated with Microvessel Density in Pancreatic Cancer. Am J Ther. 2017;24(4):e431–4.
Giannopoulos G, Pavlakis K, Parasi A, et al. The expression of matrix metalloproteinases-2 and -9 and their tissue inhibitor 2 in pancreatic ductal and ampullary carcinoma and their relation to angiogenesis and clinicopathological parameters. Anticancer Res. 2008;28(3B):1875–82.
Mroczko Barbara, Groblewska Magdalena, Okulczyk Bogna, Bogusław Kędra MS. The diagnostic value of matrix metalloproteinase 9 (MMP-9) and tissue inhibitor of matrix metalloproteinases 1 (TIMP-1) determination in the sera of colorectal adenoma and cancer patients. Int J Colorectal Dis. 2010;25(10):1177–84.
Huang L, Xu Y, Cai G, Guan Z, Cai S. Downregulation of S100A4 expression by RNA interference suppresses cell growth and invasion in human colorectal cancer cells. Oncol Rep. 2012;27(4):917–22.
Zhang G, Miyake M, Lawton A, Goodison S, Rosser CJ. Matrix metalloproteinase-10 promotes tumor progression through regulation of angiogenic and apoptotic pathways in cervical tumors. BMC Cancer. 2014;14(1):310.
Zhang W, Li Y, Yang L, et al. Knockdown of MMP-7 inhibits cell proliferation and enhances sensitivity to 5-fluorouracil and X-ray irradiation in colon cancer cells. Clin Exp Med. 2014;14(1):99–106.
Said A, Raufman J-P, Xie G. The Role of Matrix Metalloproteinases in Colorectal Cancer. Cancers. 2014;6(1):366–75.
Yang W, Arii S, Gorrin-Rivas MJ, Mori A, Onodera H, Imamura M. Human macrophage metalloelastase gene expression in colorectal carcinoma and its clinicopathologic significance. Cancer. 2001;91(7):1277–83.
Shi H, Xu JM, Hu NZ, Wang XL, Mei Q, Song YL. Transfection of mouse macrophage metalloelastase gene into murine CT-26 colon cancer cells suppresses orthotopic tumor growth, angiogenesis and vascular endothelial growth factor expression. Cancer Lett. 2006;233(1):139–50.
Rasic I, Rebic V, Rasic A, Aksamija G, Radovic S. The association of simultaneous increase in interleukin-6, C reactive protein, and matrix metalloproteinase-9 serum levels with increasing stages of colorectal cancer. J Oncol. 2018;2018:2830503.
Dragutinović VV, Radonjić NV, et al. Matrix metalloproteinase-2 (MMP-2) and -9 (MMP-9) in preoperative serum as independent prognostic markers in patients with colorectal cancer. Mol Cell Biochem. 2011;355(1–2):173–8.
Loesch M, Zhi H-Y, Hou S-W, et al. p38γ MAPK Cooperates with c-Jun in trans -Activating Matrix Metalloproteinase 9. J Biol Chem. 2010;285(20):15149–58.
Dragutinović VV, Radovanović NS, Izrael-Zivković LT, Vrvić MM. Detection of gelatinase B activity in serum of gastric cancer patients. World J Gastroenterol. 2006;12(1):105–9.
Suzuki M, Raab G, Moses MA, Fernandez CA, Klagsbrun M. Matrix metalloproteinase-3 releases active heparin-binding EGF-like growth factor by cleavage at a specific juxtamembrane site. J Biol Chem. 1997;272(50):31730–7.
Chu D, Zhang Z, Li Y, et al. Matrix metalloproteinase-9 is associated with disease-free survival and overall survival in patients with gastric cancer. Int J Cancer. 2011;129(4):887–95.
Dragutinović V, Izrael-Zivković L, Radovanović N. Relation of matrix metalloproteinase-9 to different stages of tumors in the serum of gastric cancer. Dig Dis Sci. 2009;54(6):1203–7.
Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14(2):163–76.
Krstic J, Santibanez JF. Transforming growth factor-beta and matrix metalloproteinases: functional interactions in tumor stroma-infiltrating myeloid cells. ScientificWorldJournal. 2014;2(014):521754.
Porcelli L, Iacobazzi RM, Di Fonte R, et al. CAFs and TGF-β signaling activation by mast cells contribute to resistance to Gemcitabine/Nabpaclitaxel in Pancreatic Cancer. Cancers (Basel). 2019;11(3):1–17.
Hawinkels LJAC, Verspaget HW, Van Duijn W, et al. Tissue level, activation and cellular localisation of TGF-β1 and association with survival in gastric cancer patients. Br J Cancer. 2007;97(3):398–404.
Tsushima H, Kawata S, Tamura S, et al. High levels of transforming growth factor in patients with colorectal cancer: association with disease progression. Gastroenterology. 1996;110(2):375–82.
Javle M, Li Y, Tan D, Dong X, et al. Biomarkers of TGF-β signaling pathway and prognosis of pancreatic cancer. PLoS ONE. 2014;9(1):85942.
Li X, Yue Z-C, Zhang Y-Y, et al. Elevated serum level and gene polymorphisms of TGF-β1 in gastric cancer. J Clin Lab Anal. 2008;22(3):164–71.
Emara M, Cheung P-Y, Grabowski K, Sawicki G, Wozniak M. Serum levels of matrix metalloproteinase-2 and -9 and conventional tumor markers (CEA and CA 19-9) in patients with colorectal and gastric cancers. Clin Chem Lab Med. 2009;47(8):993–1000.
Zhang Y, Yang J, Li H, Wu Y, Zhang H, Chen W. Tumor markers CA19-9, CA242 and CEA in the diagnosis of pancreatic cancer: a meta-analysis. Int J Clin Exp Med. 2015;8(7):11683–91.
Levy M, Visokai V, Lipska L, Topolcan O. Tumor markers in staging and prognosis of colorectal carcinoma. Neoplasma. 2008;55(2):138–42.
Wu C, Wu M, Chiang E, et al. Plasma Matrix Metalloproteinase-9 Level Is Better than Serum Matrix Metalloproteinase-9 Level to Predict Gastric Cancer Evolution. Clin Cancer Res. 2007;13(7):2054–60.
Acknowledgements
The authors gratefully acknowledged the medical staff of Oncology center, Mansoura University, Mansoura, Egypt, for their help in collecting patient’s data and samples for analysis.
Funding
The research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors funded the research.
Author information
Authors and Affiliations
Contributions
El-Ashmawy NE conceived the presented idea, analyzed the data and approved the manuscript in final version for publication. Khedr NF planned the experiments, wrote and reviewed the manuscript and approved it for publication. Al-Ashmawy GM designed the study, reviewed the manuscript and approved it for publication. Mansour MG conducted the research, drafted the manuscript and approved it for final approval.
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Ashmawy, N.E., Khedr, N.F., Mansour, M.G. et al. TNM staging for GIT cancers is correlated with the level of MMPs and TGF-β1. Clin Exp Med 20, 545–555 (2020). https://doi.org/10.1007/s10238-020-00651-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-020-00651-2