Abstract
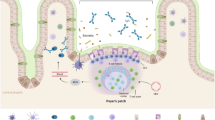
The downstream cascade of the inflammatory response to gliadin in celiac intestinal mucosa encompasses the early activation of the innate immunity that triggers the adaptive response. Therefore, the in vitro study of the pathogenic mechanism of celiac disease (CD) on enterocytes alone or mucosal T lymphocytes alone does not fully consider all the aspects of gliadin-dependent inflammation. Although the in vitro culture of specimens of intestinal mucosa obtained from celiac patients is the gold standard for the study of CD, this technique presents several technical challenges and the bioptic specimens are not easily available. So, in this paper, we described the gliadin-dependent cytokine production in a bidimensional cellular system, which is able to mimic both the innate and the adaptive steps of the mucosal immune response of CD. In the upper compartment, the intestinal epithelial cells are grown on a filter, and in the lower compartment, the mononuclear cells isolated from peripheral blood of celiac patients are cultured. Cells were apically exposed to the toxic gliadin peptide p31–43 for 3 h and then with the immunodominant gliadin fragment pα-9 for 21 h. The incubation with gliadin peptides resulted in increased levels of IL-15, INF-γ, IL-6, tumor necrosis factor (TNF)-α, IL-1β, and CCL 2, 3 and 4 in the basal supernatants, with respect to cells exposed to medium alone. The p31–43-driven epithelial priming of mucosal response consists of transglutaminase (TG2)-mediated deamidation of the immunostimulatory gliadin peptides, as demonstrated by the inhibition of pα-9 activity, when the system is exposed to blocking anti-TG2 antibody.




Similar content being viewed by others
References
Meresse B, Malamut G, Cerf-Bensussan N. Celiac disease: an immunological jigsaw. Immunity. 2012;36:907–19.
Sollid LM, Jabri B. Triggers and drivers of autoimmunity: lessons from coeliac disease. Nat Rev Immunol. 2013;13:294–302.
Jabri B, Kasarda DD, Green PH. Innate and adaptive immunity: the yin and yang of celiac disease. Immunol Rev. 2005;206:219–31.
Maiuri L, Ciacci C, Ricciardelli I, et al. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet. 2003;362:30–7.
Maiuri L, Ciacci C, Ricciardelli I, et al. Unexpected role of surface transglutaminase type II in celiac disease. Gastroenterology. 2005;129:1400–13.
Petersen J, Montserrat V, Mujico JR, et al. T-cell receptor recognition of HLA-DQ2–gliadin complexes associated with celiac disease. Nat Struct Mol Biol. 2014;21:480–8.
Tollefsen S, Arentz-Hansen H, Fleckenstein B, et al. HLA-DQ2 and -DQ8 signatures of gluten T cell epitopes in celiac disease. J Clin Invest. 2006;116:2226–36.
Sollid LM, Jabri B. Celiac disease and transglutaminase 2: a model for post-translational modification of antigens and HLA association in the pathogenesis of autoimmune disorders. Curr Opin Immunol. 2011;23:732–8.
Forsberg G, Hernell O, Melgar S, et al. Paradoxical coexpression of proinflammatory and down-regulatory cytokines in intestinal T cells in childhood celiac disease. Gastroenterology. 2002;123:667–78.
Nilsen EM, Jahnsen FL, Lundin KE, et al. Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology. 1998;115:551–63.
Garrote JA, Gómez-González E, Bernardo D, Arrantz E, Chirdo F. Celiac disease pathogenesis: the proinflammatory cytokine network. J Pediatr Gastroenterol Nutr. 2008;47:S27–32.
Nanayakkara M, Lania G, Maglio M, et al. An undigested gliadin peptide activates innate immunity and proliferative signaling in enterocytes: the role in celiac disease. Am J Clin Nutr. 2013;98:1123–35.
Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137:1912–33.
Salvati VM, Mazzarella G, Gianfrani C, et al. Recombinant human interleukin 10 suppresses gliadin dependent T cell activation in ex vivo cultured coeliac intestinal mucosa. Gut. 2005;54:46–53.
Lindfors K, Rauhavirta T, Stenman S, Mäki M, Kaukinen K. In vitro models for gluten toxicity: relevance for celiac disease pathogenesis and development of novel treatment options. Exp Biol Med. 2012;237:119–25.
Silano M, Vincentini O, Luciani A, et al. Early tissue transglutaminase-mediated response underlies K562(S)-cell gliadin—dependent agglutination. Pediatr Res. 2012;71:532–8.
De Palma G, Kamanova J, Cinova JL, et al. Modulation of phenotypic and functional maturation of dendritic cells by intestinal bacteria and gliadin: relevance for celiac disease. J Leukoc Biol. 2012;92:1043–54.
Luciani A, Villella VR, Vasaturo A, et al. Lysosomal accumulation of gliadin p31–43 peptide induces oxidative stress and tissue transglutaminase-mediated PPARgamma downregulation in intestinal epithelial cells and coeliac mucosa. Gut. 2010;59:311–9.
Barone MV, Zanzi D, Maglio M, et al. Gliadin-mediated proliferation and innate immune activation in celiac disease are due to alterations in vesicular trafficking. PLoS One. 2011;6:e17039.
Pagliari D, Cianci R, Frosali S, et al. The role of IL-15 in gastrointestinal diseases: a bridge between innate and adaptive immune response. Cytokine Growth Factor Rev. 2013;24:455–66.
DePaolo RW, Abadie V, Tang F, et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471:220–34.
Camarca A, Radano G, Di Mase R, et al. Short wheat challenge is a reproducible in-vivo assay to detect immune response to gluten. Clin Exp Immunol. 2012;169:129–36.
Lammi A, Arikoski P, Vaarala O, Kinnunen T, Ilonen J. Increased peripheral blood CD4+ T cell responses to deamidated but not to native gliadin in children with coeliac disease. Clin Exp Immunol. 2012;168:207–14.
Esposito C, Paparo F, Caputo I, et al. Expression and enzymatic activity of small intestinal tissue transglutaminase in celiac disease. Am J Gastroenterol. 2003;8:1813–20.
Tack GJ, van Wanrooij RL, Von Blomberg BM, et al. Serum parameters in the spectrum of coeliac disease: beyond standard antibody testing–a cohort study. BMC Gastroenterol. 2012;12:159.
Kalliomäki M, Satokari R, Lähteenoja H, et al. Expression of microbiota, toll-like receptors, and their regulators in the small intestinal mucosa in celiac disease. J Pediatr Gastroenterol Nutr. 2012;54:727–32.
Lammers KM, Khandelwal S, Chaudhry F, et al. Identification of a novel immunomodulatory gliadin peptide that causes interleukin-8 release in a chemokine receptor CXCR3-dependent manner only in patients with coeliac disease. Immunology. 2011;132:432–40.
Vincentini O, Quaranta MG, Viora M, Agostoni C, Silano M. Docosahexaenoic acid modulates in vitro the inflammation of celiac disease in intestinal epithelial cells via the inhibition of cPLA2. Clin Nutr. 2011;30:541–6.
Conflict of interest
None to be declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vincentini, O., Maialetti, F., Gonnelli, E. et al. Gliadin-dependent cytokine production in a bidimensional cellular model of celiac intestinal mucosa. Clin Exp Med 15, 447–454 (2015). https://doi.org/10.1007/s10238-014-0325-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-014-0325-2