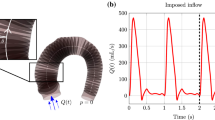
Abstract
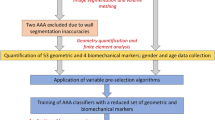
Geometric features of the aorta are linked to patient risk of rupture in the clinical decision to electively repair an ascending aortic aneurysm (AsAA). Previous approaches have focused on relationship between intuitive geometric features (e.g., diameter and curvature) and wall stress. This work investigates the feasibility of a machine learning approach to establish the linkages between shape features and FEA-predicted AsAA rupture risk, and it may serve as a faster surrogate for FEA associated with long simulation time and numerical convergence issues. This method consists of four main steps: (1) constructing a statistical shape model (SSM) from clinical 3D CT images of AsAA patients; (2) generating a dataset of representative aneurysm shapes and obtaining FEA-predicted risk scores defined as systolic pressure divided by rupture pressure (rupture is determined by a threshold criterion); (3) establishing relationship between shape features and risk by using classifiers and regressors; and (4) evaluating such relationship in cross-validation. The results show that SSM parameters can be used as strong shape features to make predictions of risk scores consistent with FEA, which lead to an average risk classification accuracy of 95.58% by using support vector machine and an average regression error of 0.0332 by using support vector regression, while intuitive geometric features have relatively weak performance. Compared to FEA, this machine learning approach is magnitudes faster. In our future studies, material properties and inhomogeneous thickness will be incorporated into the models and learning algorithms, which may lead to a practical system for clinical applications.










Similar content being viewed by others
References
Alliez P, Ucelli G, Gotsman C, Attene M (2008) Recent advances in remeshing of surfaces. In: De Floriani L, Spagnuolo M (eds) Shape analysis and structuring. Springer, Berlin, pp 53–82
Barrett JF, Keat N (2004) Artifacts in CT: recognition and avoidance. RadioGraphics 24:1679–1691. doi:10.1148/rg.246045065
Bols J, Degroote J, Trachet B, Verhegghe B, Segers P, Vierendeels J (2013) A computational method to assess the in vivo stresses and unloaded configuration of patient-specific blood vessels. J Comput Appl Math 246:10–17
Bommes D, Lévy B, Pietroni N, Puppo E, Silva C, Tarini M, Zorin D (2013) Quad-mesh generation and processing: a survey. Comput Graph Forum 32:51–76
Botsch M, Kobbelt L, Pauly M, Alliez P, Levy B (2010) Polygon mesh processing. A K Peters/CRC Press, Boca Raton
Celi S, Berti S (2014) Three-dimensional sensitivity assessment of thoracic aortic aneurysm wall stress: a probabilistic finite-element study. Eur J Cardiothorac Surg 45:467–475
Chang C-C, Lin C-J (2011) Libsvm: a library for support vector machines. ACM Trans Intell Syst Technol 2:27:21–27:27
Choke E, Cockerill G, Wilson WRW, Sayed S, Dawson J, Loftus I, Thompson MM (2005) A review of biological factors implicated in abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg 30:227–244
Coady MA, Rizzo JA, Hammond GL, Mandapati D, Darr U, Kopf GS, Elefteriades JA (1997) What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg 113:476–491
Cootes TF, Taylor CJ, Cooper DH, Graham J (1995) Active shape models—their training and application. Comput Vis Image Underst 61:38–59
Cortes C, Vapnik V (1995) Support-vector networks. Mach Learn 20:273–297
Davies RR et al (2006) Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Annal Thorac Surg 81:169–177
Davies RR, Goldstein LJ, Coady MA, Tittle SL, Rizzo JA, Kopf GS, Elefteriades JA (2002) Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Annal Thorac Surg 73:17–28
Dieleman N, van der Kolk AG, Zwanenburg JJM, Harteveld AA, Biessels GJ, Luijten PR, Hendrikse J (2014) Imaging intracranial vessel wall pathology with magnetic resonance imaging. Circulation 130:192
Doi K (2008) Computer-aided diagnosis in medical imaging: historical review, current status and future potential. Comput Med Imaging Graph 31:198–211
Doyle BJ, Callanan A, Burke PE, Grace PA, Walsh MT, Vorp DA, McGloughlin TM (2009) Vessel asymmetry as an additional diagnostic tool in the assessment of abdominal aortic aneurysms. J Vasc Surg 49:443–454
Elefteriades JA (2008) Thoracic aortic aneurysm: reading the enemy’s playbook. Yale J Biol Med 81:175–186
Elefteriades JA, Farkas EA (2010) Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol 55:841–857
Erhart P et al (2015) Finite element analysis in asymptomatic, symptomatic, and ruptured abdominal aortic aneurysms: in search of new rupture risk predictors. Eur J Vasc Endovasc Surg 49:239–245. doi:10.1016/j.ejvs.2014.11.010
Fillinger MF et al (2004) Anatomic characteristics of ruptured abdominal aortic aneurysm on conventional CT scans: implications for rupture risk. J Vasc Surg 39:1243–1252
Fillinger MF, Raghavan ML, Marra SP, Cronenwett JL, Kennedy FE (2002) In vivo analysis of mechanical wall stress and abdominal aortic aneurysm rupture risk. J Vasc Surg 36:589–597
Gasser TC (2016) Biomechanical rupture risk assessment: a consistent and objective decision-making tool for abdominal aortic aneurysm patients. AORTA 4:42–60
Gasser TC, Ogden RW, Holzapfel GA (2006) Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J R Soc Interface 3:15–35
Gee MW, Förster C, Wall WA (2010) A computational strategy for prestressing patient-specific biomechanical problems under finite deformation. Int J Numer Methods Biomed Eng 26:52–72
Georgakarakos E, Ioannou CV, Kamarianakis Y, Papaharilaou Y, Kostas T, Manousaki E, Katsamouris AN (2010) The role of geometric parameters in the prediction of abdominal aortic aneurysm wall stress. Eur J Vasc Endovasc Surg 39:42–48
Ginneken BV, Schaefer-Prokop CM, Prokop M (2011) Computer-aided diagnosis: how to move from the laboratory to the clinic. Radiology 261:719–732
Goodall C (1991) Procrustes methods in the statistical analysis of shape. J R Stat Soc Ser B (Methodol) 53:285–339
Heimann T, Meinzer H-P (2009) Statistical shape models for 3d medical image segmentation: a review. Med Image Anal 13:543–563
Hua J, Mower WR (2001) Simple geometric characteristics fail to reliably predict abdominal aortic aneurysm wall stresses. J Vasc Surg 34:308–315
Liang L, Kong F, Martin C, Pham T, Wang Q, Duncan J, Sun W (2016) Machine learning–based 3-D geometry reconstruction and modeling of aortic valve deformation using 3-D computed tomography images. Int J Numer Methods Biomed Eng 1–13. doi:10.1002/cnm.2827
Lu J, Zhou X, Raghavan ML (2007) Computational method of inverse elastostatics for anisotropic hyperelastic solids. Int J Numer Meth Eng 69:1239–1261
Maier A, Gee MW, Reeps C, Pongratz J, Eckstein H-H, Wall WA (2010) A comparison of diameter, wall stress, and rupture potential index for abdominal aortic aneurysm rupture risk prediction. Ann Biomed Eng 38:3124–3134
Martin C, Sun W, Elefteriades J (2015) Patient-specific finite element analysis of ascending aorta aneurysms. Am J Physiol Heart Circ Physiol 308:1306–1316
Martin C, Sun W, Pham T, Elefteriades J (2013) Predictive biomechanical analysis of ascending aortic aneurysm rupture potential. Acta Biomater 9:9392–9400
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, Thomas JD, ACC/AHA Task Force Members (2014) 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 129:2440–2492. doi:10.1161/CIR.0000000000000029
Pham T, Martin C, Elefteriades J, Sun W (2013) Biomechanical characterization of ascending aortic aneurysm with concomitant bicuspid aortic valve and bovine aortic arch. Acta Biomater 9:7927–7936. doi:10.1016/j.actbio.2013.04.021
Pham T, Martin C, Elefteriades J, Sun W (2013) Biomechanical characterization of ascending aortic aneurysm with concomitant bicuspid aortic valve and bovine aortic arch. Acta Biomater 9:7927–7936. doi:10.1016/j.actbio.2013.04.021
Raghavan ML, Ma B, Fillinger MF (2006) Non-invasive determination of zero-pressure geometry of arterial aneurysms. Ann Biomed Eng 34:1414–1419. doi:10.1007/s10439-006-9115-7
Raut SS, Chandra S, Shum J, Finol EA (2013) The role of geometric and biomechanical factors in abdominal aortic aneurysm rupture risk assessment. Ann Biomed Eng 41:1459–1477
Rodríguez JF, Ruiz C, Doblaré M, Holzapfel GA (2008) Mechanical stresses in abdominal aortic aneurysms: influence of diameter, asymmetry, and material anisotropy. J Biomech Eng 130:021023
Ryu C-W, Kwon O-K, Koh JS, Kim EJ (2011) Analysis of aneurysm rupture in relation to the geometric indices: aspect ratio, volume, and volume-to-neck ratio. Neuroradiology 53:883–889
Shum J, Martufi G, Martino ED, Washington CB, Grisafi J, Muluk SC, Finol EA (2011) Quantitative assessment of abdominal aortic aneurysm geometry. Ann Biomed Eng 39:277–286
Simo JC (1987) On a fully three-dimensional finite-strain viscoelastic damage model: formulation and computational aspects. Comput Methods Appl Mech Eng 60:153–173
Staib LH, Duncan JS (1996) Model-based deformable surface finding for medical images. IEEE Trans Med Imaging 15:720–731
Surazhsky V, Surazhsky T, Kirsanov D, Gortler S, Hoppe H (2005) Fast Exact and Approximate Geodesics on Meshes. ACM Trans Graph 24:553–560
Suzuki K (2012) A review of computer-aided diagnosis in thoracic and colonic imaging. Quant Imaging Med Surg 2:163–176
Umeyama S (1991) Least-squares estimation of transformation parameters between two point patterns. IEEE Trans Pattern Anal Mach Intell 13:376–380
Vande Geest JP, Wang DHJ, Wisniewski SR, Makaroun MS, Vorp DA (2006) Towards a noninvasive method for determination of patient-specific wall strength distribution in abdominal aortic aneurysms. Ann Biomed Eng 34:1098–1106. doi:10.1007/s10439-006-9132-6
Vapnik VN (1998) Statistical learning theory. Wiley-Interscience, New York
Venkatasubramaniam AK et al (2004) A comparative study of aortic wall stress using finite element analysis for ruptured and non-ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 28:168–176
Vorp DA, Raghavan ML, Webster MW (1998) Mechanical wall stress in abdominal aortic aneurysm: influence of diameter and asymmetry. J Vasc Surg 27:632–639
Weisbecker H, Pierce DM, Holzapfel GA (2014) A generalized prestressing algorithm for finite element simulations of pre-loaded geometries with application to the aorta. Int J Numer Methods Biomed Eng 30:857–872
Wittek A, Derwich W, Karatolios K, Fritzen CP, Vogt S, Schmitz-Rixen T, Blase C (2016) A finite element updating approach for identification of the anisotropic hyperelastic properties of normal and diseased aortic walls from 4d ultrasound strain imaging. J Mech Behav Biomed Mater 58:122–138. doi:10.1016/j.jmbbm.2015.09.022
Wittek A, Karatolios K, Bihari P, Schmitz-Rixen T, Moosdorf R, Vogt S, Blase C (2013) In vivo determination of elastic properties of the human aorta based on 4d ultrasound data. J Mech Behav Biomed Mater 27:167–183. doi:10.1016/j.jmbbm.2013.03.014
Wu J, Wang Y, Simon MA, Brigham JC (2012) A new approach to kinematic feature extraction from the human right ventricle for classification of hypertension: a feasibility study. Phys Med Biol 57:7905–7922
Yoshizawa S, Belyaev A, Seidel HP (2004) A fast and simple stretch-minimizing mesh parameterization. In: Proceedings of shape modeling applications, 2004, pp 200–208. doi:10.1109/SMI.2004.1314507
Acknowledgements
Research for this project was funded in part by NIH Grant R01 HL104080. Liang Liang is supported by an American Heart Association Post-doctoral Fellowship 16POST30210003.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
An Intellectual Property Disclosure has been filed on the techniques and procedures at Georgia Tech Research Corporation.
Additional information
Liang Liang and Minliang Liu have contributed equally to this work, and should be considered as co-first authors.
Appendix
Appendix
The surface remeshing method has three steps:
Step-1: Find the shortest path between a node on the left boundary and a node on the right boundary. Given a pair of nodes on the left and right boundaries, the geodesic path between them is recovered. The points on the geodesic path are on the 3D surface, but may not be the nodes of the mesh. Then a set of geodesic paths are obtained for every pair of boundary nodes, and the shortest path is selected as a cut-line. The surface mesh is cut open along the cut-line as shown in Fig. 3a, and it becomes topologically equivalent to a rectangle.
Step-2: Compute mesh-parameterization of the 3D surface mesh. The 3D surface mesh, which is cut along the cut-line, is mapped onto a 2D rectangular region, which is called mesh-parameterization. After the mapping, the 3D surface mesh is transformed to a 2D planar triangle mesh as shown in Fig. 3b.
Step-3: Divide the 2D rectangular region into a 2D quad mesh and transform it to 3D. The 2D rectangular region is discretized into a 2D mesh with rectangular elements (i.e., quad elements), as shown in Fig. 3c. Then the transform from the points of the 2D quad mesh to the 3D surface is determined by barycentric interpolation (Botsch et al. 2010) of the 2D triangle mesh. After transforming the 2D quad mesh to the 3D surface and sealing the transformed mesh along the cut-line, a 3D surface mesh with quad elements is obtained, as shown in Fig. 3d.
We utilized the exact geodesic path finding algorithm proposed by Surazhsky et al. (2005), for Step-1. Based on the work of Yoshizawa et al. (2004), we developed a stretch-minimizing-based algorithm for Step-2, and it has two stages:
Stage-1 of Step-2: Find an initialization mesh-parameterization based on barycentric mapping and mean value theorem (Botsch et al. 2010). Barycentric mapping is used to build a parameterization of the 3D triangle surface mesh, i.e., transforming the 3D surface mesh to a 2D planar triangle mesh. The boundary of the 2D planar mesh forms a rectangle. Each triangle \({\varvec{P}}_i =( {{\varvec{p}}_1 ,{\varvec{p}}_2 ,{\varvec{p}}_3 } )\) of the 3D surface mesh is mapped to a triangle \({\varvec{Q}}_i =({{\varvec{q}}_1 ,{\varvec{q}}_2 ,{\varvec{q}}_3 })\) of the 2D planar mesh. Each node \({\varvec{p}}_i =[{x_i ,y_i ,z_i }]\) of the 3D surface mesh is mapped to a node \({\varvec{q}}_i =[{u_i ,v_i } ]\) of the 2D planar mesh. Here \([{x_i ,y_i ,z_i }]\) denotes 3D coordinate, and \([{u_i ,v_i }]\) denotes 2D coordinate. Based on barycentric mapping, the node coordinates of the 2D planar mesh are determined by
where M is the number of interior nodes and N is the total number of nodes. By using the mean value theorem, each coefficient is determined by
where \(a_{i,j} >0\) if \({\varvec{p}}_i \) and \({\varvec{p}}_j\) are connected by an edge, otherwise \(a_{i,i} =-\mathop \sum \nolimits _{j\ne i} a_{i,j} \) and \(a_{i,j} =0\). \(\theta _{i,j} \) and \(\delta _{i,j} \) are angles between the edge from \({\varvec{p}}_i \) to \({\varvec{p}}_j \) and its two adjacent edges, respectively. After the coefficients \(\{ {a_{i,j} }\}\) are calculated, the node coordinates of the 2D planar mesh are obtained by solving Eq. (1).
Now, an inverse transform from a point on the 2D plane to the 3D surface can be obtained: let \({\varvec{q}}\) be a point inside \({\varvec{Q}}_i \); then, its corresponding point \({\varvec{p}}\) on the 3D surface is determined by an affine mapping, namely barycentric interpolation:
where \(\langle {{\varvec{q}}_a , {\varvec{q}}_b ,{\varvec{q}}_c } \rangle \) is the area of the triangle defined by the three points.
Stage-2 of Step-2: Refine the mesh-parameterization based on stretch minimization. After Stage-1, the 3D surface mesh is mapped onto a 2D parametric plane, resulting a 2D planar mesh composed of the same number of nodes and triangle elements. The goal of this refinement stage is to change the node coordinates of the 2D planar mesh such that mesh distortion is minimized. Mesh distortion is measured by the average stretch \(\upmu \), given by
where \(A({{\varvec{P}}_i })\) denote the area of the triangle \({\varvec{P}}_i ; \upmu _{P_i }\) is the local stretch associated with triangle \({\varvec{P}}_i\), and it is defined as
where \({\Gamma }\) is max eigenvalue and \({\Gamma }\) is the min eigenvalue of the deformation gradient tensor derived from the affine mapping (Eq. 3). We utilize the algorithm proposed by Yoshizawa et al. (2004) to find the optimal node coordinates such that the average stretch \(\upmu \) is minimized. This algorithm has two iteration steps:
-
(1)
Update the node coordinates of the 2D triangle mesh by minimizing the local energy function
$$\begin{aligned} E=\mathop \sum \limits _j a_{i,j} {\Vert \varvec{q}}_i -{\varvec{q}}_j\Vert ^{2} \end{aligned}$$(13)In this step, the coefficients \(\left\{ {a_{i,j} } \right\} \) are fixed. The solution of this minimization problem is found by solving a set of linear equations.
-
(2)
Update each coefficient by using each local stretch
$$\begin{aligned} a_{i,j} \leftarrow \frac{a_{i,j} }{\upmu _{P_j } } \end{aligned}$$(14)The initial values of the coefficients are obtained in Stage-1.
After a few iterations, the average stretch \(\upmu \) will be reduced. Then the rectangle region is discretized to a 2D planar quad mesh as shown in Fig. 3c. Using the affine mapping (Eq. 3) each node of the 2D planar quad mesh is transformed to the 3D surface. As a result, the 3D surface is now represented by a quad mesh as shown in Fig. 3d.
Rights and permissions
About this article
Cite this article
Liang, L., Liu, M., Martin, C. et al. A machine learning approach to investigate the relationship between shape features and numerically predicted risk of ascending aortic aneurysm. Biomech Model Mechanobiol 16, 1519–1533 (2017). https://doi.org/10.1007/s10237-017-0903-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-017-0903-9