Abstract
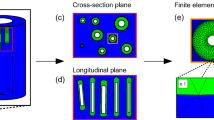
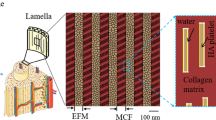
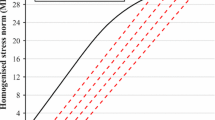
Bone is a complex material which exhibits several hierarchical levels of structural organization. At the submicron-scale, the local tissue porosity gives rise to discontinuities in the bone matrix which have been shown to influence damage behavior. Computational tools to model the damage behavior of bone at different length scales are mostly based on finite element (FE) analysis, with a range of algorithms developed for this purpose. Although the local mechanical behavior of bone tissue is influenced by microstructural features such as bone canals and osteocyte lacunae, they are often not considered in FE damage models due to the high computational cost required to simulate across several length scales, i.e., from the loads applied at the organ level down to the stresses and strains around bone canals and osteocyte lacunae. Hence, the aim of the current study was twofold: First, a multilevel FE framework was developed to compute, starting from the loads applied at the whole bone scale, the local mechanical forces acting at the micrometer and submicrometer level. Second, three simple microdamage simulation procedures based on element removal were developed and applied to bone samples at the submicrometer-scale, where cortical microporosity is included. The present microdamage algorithm produced a qualitatively analogous behavior to previous experimental tests based on stepwise mechanical compression combined with in situ synchrotron radiation computed tomography. Our results demonstrate the feasibility of simulating microdamage at a physiologically relevant scale using an image-based meshing technique and multilevel FE analysis; this allows relating microdamage behavior to intracortical bone microstructure.










Similar content being viewed by others
References
Arbenz P, van Lenthe GH et al (2008) A scalable multi-level preconditioner for matrix-free mu-finite element analysis of human bone structures. Int J Numer Meth Eng 73(7):927–947
Bayraktar HH, Morgan EF et al (2004) Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J Biomech 37(1):27–35
Bell KL, Loveridge N et al (1999) Regional differences in cortical porosity in the fractured femoral neck. Bone 24(1):57–64
Belytschko T, Black T (1999) Elastic crack growth in finite elements with minimal remeshing. Int J Numer Meth Eng 45(5):601–620
Bonivtch AR, Bonewald LF et al (2007) Tissue strain amplification at the osteocyte lacuna: a microstructural finite element analysis. J Biomech 40(10):2199–2206
Bordas S, Moran B (2006) Enriched finite elements and level sets for damage tolerance assessment of complex structures. Eng Fract Mech 73(9):1176–1201
Bousson V, Peyrin F et al (2004) Cortical bone in the human femoral neck: three-dimensional appearance and porosity using synchrotron radiation. J Bone Miner Res 19(5):794–801
Budyn E, Hoc T (2010) Analysis of micro fracture in human Haversian cortical bone under transverse tension using extended physical imaging. Int J Numer Meth Eng 82(8):940–965
Budyn E, Hoc T et al (2008) Fracture strength assessment and aging signs detection in human cortical bone using an X-FEM multiple scale approach. Comput Mech 42(4):579–591
Burr DB, Forwood MR et al (1997) Bone microdamage acid skeletal fragility in osteoporotic and stress fractures. J Bone Miner Res 12(1):6–15
Christen D, Levchuk A et al (2012) Deformable image registration and 3D strain mapping for the quantitative assessment of cortical bone microdamage. J Mech Behav Biomed Mater 8:184–193
Christen D, Webster DJ et al (2010) Multiscale modelling and nonlinear finite element analysis as clinical tools for the assessment of fracture risk. Philos Trans R Soc Math Phys Eng Sci 368(1920):2653–2668
Cooper DML, Turinsky AL et al (2003) Quantitative 3D analysis of the canal network in cortical bone by micro-computed tomography. Anat Rec 274B(1):169–179
Cristofolini L, Taddei F et al (2008) Multiscale investigation of the functional properties of the human femur. Philos Trans R Soc Math A Phys Eng Sci 366(1879):3319–3341
Deligianni DD, Apostolopoulos CA (2008) Multilevel finite element modeling for the prediction of local cellular deformation in bone. Biomech Model Mechanobiol 7(2):151–159
Donaldson FE, Pankaj P et al (2011) Relating age and micro-architecture with apparent-level elastic constants: a micro-finite element study of female cortical bone from the anterior femoral midshaft. Proc Inst Mech Eng Part H J Eng Med 225(H6):585–596
Donaldson FE, Pankaj P et al (2008) Virtual trabecular bone models and their mechanical response. Proc Inst Mech Eng Part H J Eng Med 222(H8):1185–1195
Eswaran SK, Gupta A et al (2006) Cortical and trabecular load sharing in the human vertebral body. J Bone Miner Res 21(2):307–314
Faridani A, Finch DV et al (1997) Local tomography. SIAM J Appl Math 57(4):1095–1127
Fratzl P, Gupta HS et al (2007) Hindered crack propagation in materials with periodically varying Young’s modulus: lessons from biological materials. Adv Mater 19(18):2657
Fratzl P, Weinkamer R (2007) Nature’s hierarchical materials. Prog Mater Sci 52(8):1263–1334
Ghanbari J, Naghdabadi R (2009) Nonlinear hierarchical multiscale modeling of cortical bone considering its nanoscale microstructure. J Biomech 42(10):1560–1565
Gravouil A, Moes N et al (2002) Non-planar 3D crack growth by the extended finite element and level sets, Part II: level set update. Int J Numer Meth Eng 53(11):2569–2586
Hambli R (2013) Micro-CT finite element model and experimental validation of trabecular bone damage and fracture. Bone 56(2): 363–374
Hardisty MR, Zauel R et al (2013) The importance of intrinsic damage properties to bone fragility: a finite element study. J Biomech Eng Trans Asme 135(1):011004
Jager I, Fratzl P (2000) Mineralized collagen fibrils: a mechanical model with a staggered arrangement of mineral particles. Biophys J 79(4):1737–1746
Ji BH, Gao HJ (2006) Elastic properties of nanocomposite structure of bone. Compos Sci Technol 66(9):1212–1218
Jones AC, Sheppard AP et al (2004) Three-dimensional analysis of cortical bone structure using X-ray micro-computed tomography. Phys A Stat Mech Appl 339(1–2):125–130
Jordan GR, Loveridge N et al (2000) Spatial clustering of remodeling osteons in the femoral neck cortex: a cause of weakness in hip fracture? Bone 26(3):305–313
Keaveny TM, Morgan EF et al (2001) Biomechanics of trabecular bone. Annu Rev Biomed Eng 3:307–333
Kerschnitzki M, Wagermaier W et al (2011) The organization of the osteocyte network mirrors the extracellular matrix orientation in bone. J Struct Biol 173(2):303–311
Koester KJ, Ager JW et al (2008) The true toughness of human cortical bone measured with realistically short cracks. Nat Mater 7(8):672–677
Lee TC, Mohsin S et al (2003) Detecting microdamage in bone. J Anat 203(2):161–172
McCalden RW, McGeough JA et al (1993) Age-related-changes in the tensile properties of cortical bone: the relative importance of changes in porosity, mineralization, and microstructure. J Bone Joint Surg Am 75A(8):1193–1205
Moes N, Dolbow J et al (1999) A finite element method for crack growth without remeshing. Int J Numer Method Eng 46(1):131–150
Moes N, Gravouil A et al (2002) Non-planar 3D crack growth by the extended finite element and level sets, Part I: mechanical model. Int J Numer Method Eng 53(11):2549–2568
Nalla RK, Stolken JS et al (2005) Fracture in human cortical bone: local fracture criteria and toughening mechanisms. J Biomech 38(7):1517–1525
Needleman A (1987) A continuum model for void nucleation by inclusion debonding. J Appl Mech Trans Asme 54(3):525–531
Nicolella DP, Moravits DE et al (2006) Osteocyte lacunae tissue strain in cortical bone. J Biomech 39(9):1735–1743
Niebur GL, Feldstein MJ et al (2000) High-resolution finite element models with tissue strength asymmetry accurately predict failure of trabecular bone. J Biomech 33(12):1575–1583
O’Brien FJ, Taylor D et al (2003) Microcrack accumulation at different intervals during fatigue testing of compact bone. J Biomech 36(7):973–980
Pankaj P, Donaldson FE (2013) Algorithms for a strain-based plasticity criterion for bone. Int J Numer Methods Biomed Eng 29(1):40–61
Peterlik H, Roschger P et al (2006) From brittle to ductile fracture of bone. Nat Mater 5(1):52–55
Riks E (1979) An incremental approach to the solution of snapping and buckling problems. Int J Solids Struct 15(7):529–551
Ritchie RO, Buehler MJ et al (2009) Plasticity and toughness in bone. Phys Today 62(6):41–47
Ruffoni D, Kohler T et al (2013) High-throughput quantification of the mechanical competence of murine femora: a highly automated approach for large-scale genetic studies. Bone 55(3):216–221
Ruffoni D, van Lenthe GH (2010) Chapter 93: finite element analysis in bone research: a computational method relating structure to mechanical function. Comprehensive biomaterials. Elsevier Science
Ruffoni D, Wirth AJ et al (2012) The different contributions of cortical and trabecular bone to implant anchorage in a human vertebra. Bone 50(3):733–738
Sahar ND, Hong SI et al (2005) Micro- and nano-structural analyses of damage in bone. Micron 36(7–8):617–629
Schneider P, Levchuk A et al (2010a) Automated micro-compression device for dynamic image-guided failure assessment of bone ultrastructure and bone microdamage. Biomed Eng 55(Suppl 1):8–10
Schneider P, Meier M et al (2010b) Towards quantitative 3D imaging of the osteocyte lacuno-canalicular network. Bone 47(5):848–858
Schneider P, Meier M et al (2011) Serial FIB/SEM imaging for quantitative 3D assessment of the osteocyte lacuno-canalicular network. Bone 49(2):304–311
Schneider P, Stauber M et al (2007) Ultrastructural properties in cortical bone vary greatly in two inbred strains of mice as assessed by synchrotron light based micro- and Nano-CT. J Bone Miner Res 22(10):1557–1570
Schneider P, Voide R et al (2013) The importance of the intracortical canal network for murine bone mechanics. Bone 53(1):120–128
Schneider P, Voide R et al (2009) Post-processing technique for improved assessment of hard tissues in the submicrometer domain using local synchrotron radiation-based computed tomography. Biomed Technol 54(1):48–54
Schulte FA, Ruffoni D et al (2013) Local mechanical stimuli regulate bone formation and resorption in mice at the tissue level. Plos One 8(4):e62172
Taylor D, Hazenberg JG et al (2007) Living with cracks: damage and repair in human bone. Nat Mater 6(4):263–268
Tomar V (2008) Modeling of dynamic fracture and damage in two-dimensional trabecular bone microstructures using the cohesive finite element method. J Biomech Eng Trans Asme 130(2):021021
Ural A (2009) Prediction of Colles’ fracture load in human radius using cohesive finite element modeling. J Biomech 42(1):22–28
Ural A, Vashishth D (2007) Effects of intracortical porosity on fracture toughness in aging human bone: a mu CT-based cohesive finite element study. J Biomech Eng Trans Asme 129(5):625–631
van Rietbergen B, Weinans H et al (1995) A new method to determine trabecular bone elastic properties and loading using micromechanical finite-element models. J Biomech 28(1):69
Vashishth D, Koontz J et al (2000) In vivo diffuse damage in human vertebral trabecular bone. Bone 26(2):147–152
Vaughan TJ, McCarthy CT et al (2012) A three-scale finite element investigation into the effects of tissue mineralisation and lamellar organisation in human cortical and trabecular bone. J Mech Behav Biomed Mater 12:50–62
Verhulp E, van Rietbergen B et al (2008a) Indirect determination of trabecular bone effective tissue failure properties using micro-finite element simulations. J Biomech 41(7):1479–1485
Verhulp E, Van Rietbergen B et al (2008b) Micro-finite element simulation of trabecular-bone post-yield behaviour: effects of material model, element size and type. Comput Methods Biomech Biomed Eng 11(4):389–395
Voide R, Schneider P et al (2011) The importance of murine cortical bone microstructure for microcrack initiation and propagation. Bone 49(6):1186–1193
Voide R, Schneider P et al (2009) Time-lapsed assessment of microcrack initiation and propagation in murine cortical bone at submicrometer resolution. Bone 45(2):164–173
Voide R, van Lenthe GH et al (2008) Bone morphometry strongly predicts cortical bone stiffness and strength, but not toughness, in inbred mouse models of high and low bone mass. J Bone Miner Res 23(8):1194–1203
Yeni YN, Brown CU et al (1997) The influence of bone morphology on fracture toughness of the human femur and tibia. Bone 21(5):453–459
Zimmermann EA, Launey ME et al (2010) The significance of crack-resistance curves to the mixed-mode fracture toughness of human cortical bone. Biomaterials 31(20):5297–5305
Zimmermann EA, Schaible E et al (2011) Age-related changes in the plasticity and toughness of human cortical bone at multiple length scales. Proc Natl Acad Sci USA 108(35):14416–14421
Zysset PK, Curnier A (1996) A 3D damage model for trabecular bone based on fabric tensors. J Biomech 29(12):1549–1558
Acknowledgments
The authors acknowledge the Swiss National Supercomputing Centre (CSCS, Lugano, Switzerland). F.D. gratefully acknowledges support from the Carnegie Trust for the Universities of Scotland, P.S. from the Swiss National Science Foundation and A.L. from the Whitaker Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Donaldson, F., Ruffoni, D., Schneider, P. et al. Modeling microdamage behavior of cortical bone. Biomech Model Mechanobiol 13, 1227–1242 (2014). https://doi.org/10.1007/s10237-014-0568-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-014-0568-6