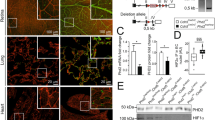
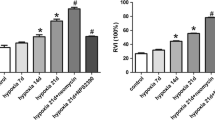
Abstract
Proximal pulmonary artery (PA) stiffening is a strong predictor of mortality in pulmonary hypertension. Collagen accumulation is mainly responsible for PA stiffening in hypoxia-induced pulmonary hypertension (HPH) in mouse models. We hypothesized that collagen cross-linking and the type I isoform are the main determinants of large PA mechanical changes during HPH, which we tested by exposing mice that resist type I collagen degradation (Col1a1\(^\mathrm{R/R})\) and littermate controls (Col1a1\(^{+/+})\) to hypoxia for 10 days with or without \(\beta \)-aminopropionitrile (BAPN) treatment to prevent cross-link formation. Static and dynamic mechanical tests were performed on isolated PAs with smooth muscle cells (SMC) in passive and active states. Percentages of type I and III collagen were quantified by histology; total collagen content and cross-linking were measured biochemically. In the SMC passive state, for both genotypes, hypoxia tended to increase PA stiffness and damping capacity, and BAPN treatment limited these increases. These changes were correlated with collagen cross-linking (\(p<0.05\)). In the SMC active state, hypoxia increased PA dynamic stiffness and BAPN had no effect in Col1a1\(^{+/+}\) mice (\(p<0.05\)). PA stiffness did not change in Col1a1\(^\mathrm{R/R}\) mice. Similarly, damping capacity did not change for either genotype. Type I collagen accumulated more in Col1a1\(^{+/+}\) mice, whereas type III collagen increased more in Col1a1\(^\mathrm{R/R}\) mice during HPH. In summary, PA passive mechanical properties (both static and dynamic) are related to collagen cross-linking. Type I collagen turnover is critical to large PA remodeling during HPH when collagen metabolism is not mutated and type III collagen may serve as a reserve.






Similar content being viewed by others
References
Armentano RL, Barra JG, Pessana FM, Craiem DO, Graf S, Santana DB, Sanchez RA (2007) Smart smooth muscle spring-dampers. Smooth muscle smart filtering helps to more efficiently protect the arterial wall. IEEE Eng Med Biol Mag 26(1):62–70
Armentano RL, Levenson J, Barra JG, Fischer EI, Breitbart GJ, Pichel RH, Simon A (1991) Assessment of elastin and collagen contribution to aortic elasticity in conscious dogs. Am J Physiol 260(6 Pt 2):H1870–1877
Baker AM, Bird D, Lang G, Cox TR, Erler JT (2012) Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene. doi:10.1038/onc.2012.202
Barbera JA, Peinado VI, Santos S (2003) Pulmonary hypertension in chronic obstructive pulmonary disease. Eur Respir J 21(5):892–905
Barra JG, Armentano RL, Levenson J, Fischer EI, Pichel RH, Simon A (1993) Assessment of smooth muscle contribution to descending thoracic aortic elastic mechanics in conscious dogs. Circ Res 73(6):1040–1050
Berry CL, Greenwald SE, Menahem N (1981) Effect of beta-aminopropionitrile on the static elastic properties and blood pressure of spontaneously hypertensive rats. Cardiovasc Res 15(7):373–381
Boutouyrie P, Boumaza S, Challande P, Lacolley P, Laurent S (1998) Smooth muscle tone and arterial wall viscosity: an in vivo/in vitro study. Hypertension 32(2):360–364
Bruel A, Ortoft G, Oxlund H (1998) Inhibition of cross-links in collagen is associated with reduced stiffness of the aorta in young rats. Atherosclerosis 140(1):135–145
Carroll CC, Whitt JA, Peterson A, Gump BS, Tedeschi J, Broderick TL (2012) Influence of acetaminophen consumption and exercise on Achilles tendon structural properties in male Wistar rats. Am J Physiol Regul Integr Comp Physiol 302(8):R990–995. doi:10.1152/ajpregu.00659.2011
Chesler NC, Thompson-Figueroa J, Millburne K (2004) Measurements of mouse pulmonary artery biomechanics. J Biomech Eng 126(2):309–314
Drexler ES, Bischoff JE, Slifka AJ, McCowan CN, Quinn TP, Shandas R, Ivy DD, Stenmark KR (2008) Stiffening of the extrapulmonary arteries from rats in chronic hypoxic pulmonary hypertension. J Res Natl Inst Stand Technol 113(4):239–249
Efron B, Tibshirani R (1993) An introduction to the Bootstrap. Chapman & Hall/CRC, Boca Raton
Estrada KD, Chesler NC (2009) Collagen-related gene and protein expression changes in the lung in response to chronic hypoxia. Biomech Model Mechanobiol 8(4):263–272. doi:10.1007/s10237-008-0133-2
Fraser KL, Tullis DE, Sasson Z, Hyland RH, Thornley KS, Hanly PJ (1999) Pulmonary hypertension and cardiac function in adult cystic fibrosis: role of hypoxemia. Chest 115(5):1321–1328
Fung YC (1991) What are the residual stresses doing in our blood vessels? Ann Biomed Eng 19(3):237–249
Fung YC, Liu SQ (1991) Changes of zero-stress state of rat pulmonary arteries in hypoxic hypertension. J Appl Physiol 70(6):2455–2470
Gan CT, Lankhaar JW, Westerhof N, Marcus JT, Becker A, Twisk JW, Boonstra A, Postmus PE, Vonk-Noordegraaf A (2007) Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest 132(6):1906–1912. doi:10.1378/chest.07-1246
Goodman LA (1962) The variance of the product of K random variables. J Am Stat Assoc 57:54–60
Halme T, Peltonen J, Sims TJ, Vihersaari T, Penttinen R (1986) Collagen in human aorta. Changes in the type III/I ratio and concentration of the reducible crosslink, dehydrohydroxylysinonorleucine in ascending aorta from healthy subjects of different age and patients with annulo-aortic ectasia. Biochim Biophys Acta 881(2):222–228
Henkel W, Glanville RW (1982) Covalent crosslinking between molecules of type I and type III collagen. The involvement of the N-terminal, nonhelical regions of the alpha 1 (I) and alpha 1 (III) chains in the formation of intermolecular crosslinks. Eur J Biochem 122(1):205–213
Hiestand D, Phillips B (2008) The overlap syndrome: chronic obstructive pulmonary disease and obstructive sleep apnea. Crit Care Clin 24(3):551–563, vii. doi:10.1016/j.ccc.2008.02.005
Hudetz AG (1979) Incremental elastic modulus for orthotropic incompressible arteries. J Biomech 12(9):651–655. doi:10.1016/0021-9290(79)90015-0
Hultgardh-Nilsson A, Durbeej M (2007) Role of the extracellular matrix and its receptors in smooth muscle cell function: implications in vascular development and disease. Curr Opin Lipidol 18(5):540–545. doi:10.1097/MOL.0b013e3282ef77e900041433-200710000-00010
Junqueira LC, Cossermelli W, Brentani R (1978) Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch Histol Jpn 41(3):267–274
Kerr JS, Riley DJ, Frank MM, Trelstad RL, Frankel HM (1984) Reduction of chronic hypoxic pulmonary hypertension in the rat by beta-aminopropionitrile. J Appl Physiol 57(6):1760–1766
Kerr JS, Ruppert CL, Tozzi CA, Neubauer JA, Frankel HM, Yu SY, Riley DJ (1987) Reduction of chronic hypoxic pulmonary hypertension in the rat by an inhibitor of collagen production. Am Rev Respir Dis 135(2):300–306
Kobs RW, Chesler NC (2006) The mechanobiology of pulmonary vascular remodeling in the congenital absence of eNOS. Biomech Model Mechanobiol 5(4):217–225. doi:10.1007/s10237-006-0018-1
Kobs RW, Muvarak NE, Eickhoff JC, Chesler NC (2005) Linked mechanical and biological aspects of remodeling in mouse pulmonary arteries with hypoxia-induced hypertension. Am J Physiol Heart Circ Physiol 288(3):H1209–1217. doi:10.1152/ajpheart.01129.2003
Kontadakis GA, Ginis H, Karyotakis N, Pennos A, Pentari I, Kymionis GD, Pallikaris IG (2012) In vitro effect of corneal collagen cross-linking on corneal hydration properties and stiffness. Graefes Arch Clin Exp Ophthalmol. doi:10.1007/s00417-012-2082-9
Lakes RS (1999) Viscoelastic solids. CRC Press LLC, Boca Raton
Leushner JR, Haust MD (1986) Interstitial collagens in fibrous atherosclerotic lesions of human aorta. Pathol Biol (Paris) 34(1):14–18
Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD (2006) Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol 47(4):799–803. doi:10.1016/j.jacc.2005.09.054
Mavrilas D, Sinouris EA, Vynios DH, Papageorgakopoulou N (2005) Dynamic mechanical characteristics of intact and structurally modified bovine pericardial tissues. J Biomech 38(4):761–768. doi:10.1016/j.jbiomech.2004.05.019
Miller EJ, Furuto DK, Narkates AJ (1991) Quantitation of type I, III, and V collagens in human tissue samples by high-performance liquid chromatography of selected cyanogen bromide peptides. Anal Biochem 196(1):54–60
Moiseeva EP (2001) Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc Res 52(3):372–386
Namba T, Tsutsui H, Tagawa H, Takahashi M, Saito K, Kozai T, Usui M, Imanaka-Yoshida K, Imaizumi T, Takeshita A (1997) Regulation of fibrillar collagen gene expression and protein accumulation in volume-overloaded cardiac hypertrophy. Circulation 95(10):2448–2454
Ooi CY, Wang Z, Tabima DM, Eickhoff JC, Chesler NC (2010) The role of collagen in extralobar pulmonary artery stiffening in response to hypoxia-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 299(6):H1823–1831. doi:10.1152/ajpheart.00493.2009
Poiani GJ, Tozzi CA, Choe JK, Yohn SE, Riley DJ (1990a) An antifibrotic agent reduces blood pressure in established pulmonary hypertension in the rat. J Appl Physiol 68(4):1542–1547
Poiani GJ, Tozzi CA, Yohn SE, Pierce RA, Belsky SA, Berg RA, Yu SY, Deak SB, Riley DJ (1990b) Collagen and elastin metabolism in hypertensive pulmonary arteries of rats. Circ Res 66(4):968–978
Rich L, Whittaker P (2005) Collagen and picrosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci 22(2):97–104
Santana DB, Barra JG, Grignola JC, Gines FF, Armentano RL (2005) Pulmonary artery smooth muscle activation attenuates arterial dysfunction during acute pulmonary hypertension. J Appl Physiol 98(2):605–613. doi:10.1152/japplphysiol.00361.2004
Sims TJ, Rasmussen LM, Oxlund H, Bailey AJ (1996) The role of glycation cross-links in diabetic vascular stiffening. Diabetologia 39(8):946–951
Stegemann JP, Hong H, Nerem RM (2005) Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J Appl Physiol 98(6):2321–2327. doi:10.1152/japplphysiol.01114.2004
Stenmark KR, Fagan KA, Frid MG (2006) Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99(7):675–691. doi:10.1161/01.RES.0000243584.45145.3f
Tabima DM, Chesler NC (2010) The effects of vasoactivity and hypoxic pulmonary hypertension on extralobar pulmonary artery biomechanics. J Biomech 43(10):1864–1869. doi:10.1016/j.jbiomech.2010.03.033
Tian L, Lammers SR, Kao PH, Albietz JA, Stenmark KR, Qi HJ, Shandas R, Hunter KS (2012) Impact of residual stretch and remodeling on collagen engagement in healthy and pulmonary hypertensive calf pulmonary arteries at physiological pressures. Ann Biomed Eng 40(7):1419–1433. doi:10.1007/s10439-012-0509-4
Tozzi CA, Christiansen DL, Poiani GJ, Riley DJ (1994) Excess collagen in hypertensive pulmonary arteries decreases vascular distensibility. Am J Respir Crit Care Med 149(5):1317–1326
Wagner HP, Humphrey JD (2011) Differential passive and active biaxial mechanical behaviors of muscular and elastic arteries: basilar versus common carotid. J Biomech Eng 133(5):051009. doi:10.1115/1.4003873
Wang Z, Chesler NC (2011) Role of collagen content and cross-linking in large pulmonary arterial stiffening after chronic hypoxia. Biomech Model Mechanobiol 11(1–2):279–289. doi:10.1007/s10237-011-0309-z
Whittaker P, Kloner RA, Boughner DR, Pickering JG (1994) Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol 89(5):397–410
Zhao W, Byrne MH, Boyce BF, Krane SM (1999) Bone resorption induced by parathyroid hormone is strikingly diminished in collagenase-resistant mutant mice. J Clin Invest 103(4):517–524. doi:10.1172/JCI5481
Acknowledgments
This study is supported by NIH R01 HL086939 (NCC) and AHA Midwest Affiliate Postdoctoral Fellowship 10POST2640148 (ZW).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Z., Lakes, R.S., Eickhoff, J.C. et al. Effects of collagen deposition on passive and active mechanical properties of large pulmonary arteries in hypoxic pulmonary hypertension. Biomech Model Mechanobiol 12, 1115–1125 (2013). https://doi.org/10.1007/s10237-012-0467-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-012-0467-7