Abstract
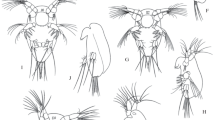
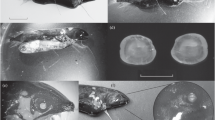
Cyclopoid copepods are common in lakes and ponds, and they have a significant predation impact on the communities of the small zooplankton species. To reduce the predation risk, some cladoceran zooplankters develop protuberant (defensive) morphologies in the presence of the copepods. In the case of the small cladoceran Bosmina, they elongate their appendages (antennule and mucrone) and change the antennule morphotype. However, information about the effectiveness of these defensive devices against copepod predation is still insufficient. In our study, to find the compositive effects of these appendages on the vulnerability of Bosmina, we exposed two bosminid species (B. longirostris and B. fatalis) of different body sizes and with appendages of different lengths and shapes to copepod (Mesocyclops) predation. The experiment revealed that the shape of the antennule is a main factor determining the bosminid’s vulnerability to copepod predation and indicated that the protection of the opened ventral carapace must be a key strategy by which Bosmina avoids copepod predation.




Similar content being viewed by others
References
Brandl Z (1998) Life strategy and feeding relations of Cyclops vicinus in two reservoirs. Int Rev Hydrobiol 83:381–388
Caramujo MJ, Boavida MJ (2000) Induction and costs of tail spine elongation in Daphnia hyalina × galeata: reduction of susceptibility to copepod predation. Freshw Biol 45:413–423
Chang KH, Hanazato T (2002) Morphological defense of Bosmina fatalis against invertebrate predators in Lake Suwa. Verh Int Verein Limnol 28:1279–1283
Chang KH, Hanazato T (2003a) Seasonal and reciprocal succession and cyclomorphosis of two Bosmina species (Cladocera, Crustacea) co-existing in a lake: their relationship with invertebrate predators. J Plankton Res 25:141–150
Chang KH, Hanazato T (2003b) Vulnerability of cladoceran species to predation by the copepod Mesocyclops leuckarti: laboratory observations on the behavioural interactions between predator and prey. Freshw Biol 48:476–484
Chang KH, Hanazato T (2005) Prey handling time and ingestion probability for Mesocyclops sp. predation on small cladoceran species Bosmina longirostris, Bosminopsis deitersi, and Scapholeberis mucronata. Limnology 6:39–44
Enz CA, Heller C, Müller R, Bürgi HR (2001) Investigations on fecundity of Bythotrophes longimanus l in Lake Lucerne (Switzerland) and on niche segregation of Leptodora kindti and Bythotrophes longimanus in Swiss lakes. Hydrobiologia 464:143–151
Gliwicz ZM (2002) On the different nature of top-down and bottom-up effects in pelagic food webs. Freshw Biol 47:2296–2312
Gliwicz ZM, Lampert W (1993) Body-size related survival of cladocerans in a trophic gradient: an enclosure study. Arch Hydrobiol 129:1–23
Gliwicz ZM, Umana U (1994) Cladoceran body size and vulnerability to copepod predation. Limnol Oceanogr 32:419–424
Greene CH (1983) Selective predation in freshwater zooplankton communities. Int Rev Gesamten Hydrobiol 68:297–315
Hanazato T, Yasuno M (1989) Zooplankton community structure driven by vertebrate and invertebrate predators. Oecologia 81:450–458
Herzig A (1995) Leptodora kindti: efficient predator and preferred prey item in Neusiedler See, Austria. Hydrobiologia 307:273–282
Herzig A, Auer B (1990) The feeding behavior of Leptodora kindtii and its impact on the zooplankton community of Neusiedler See (Austria). Hydrobiologia 198:107–117
Kappes H, Sinsch U (2002) Morphological variation in Bosmina longirostris (O.F. Muller, 1785) (Crustacea: Cladocera): consequence of cyclomorphosis or indication of cryptic species? J Zool Syst Evol Res 40:113–122
Kerfoot WC (1977) Implications of copepod predation. Limnol Oceanogr 22:316–325
Kerfoot WC (1978) Combat between predatory copepods and their prey: Cyclops, Epischura, and Bosmina. Limnol Oceanogr 22:316–325
Kerfoot WC (1987) Translocation experiment: Bosmina responses to copepod predation. Ecology 68:596–610
Kerfoot WC (1988) Defensive spines: inverse relationship between coefficients of variation and size. Limnol Oceanogr 33:1412–1429
Lagergren R, Hellsten M, Stenson JAE (1997) Increased drag, and thus lower speed: a cost for morphological defence in Bosmina (Eubosmina) (Crustacea: Cladocera). Funct Ecol 11:484–488
Lampert W (1978) Climatic conditions and planktonic interactions as factors controlling the regular succession of spring algal bloom and extremely clear water in Lake Constance. Verh Int Verein Limnol 20:969–974
Li JL, Li HW (1979) Species-specific factors affecting predator–prey interactions of the copepod Acanthocyclops vernalis with its natural prey. Limnol Oceanogr 24:613–626
Lunte C, Luecke C (1990) Trophic interactions of Leptodora in Lake Mendota. Limnol Oceanogr 35:1091–1100
Manca M, Comoli P (1995) Seasonal changes in size of feeding basket of Leptodora kindtii (Focke) in Lago Maggiore as related to variations in prey size selection. Limnol Oceanogr 40:834–838
Nagata T, Hanazato T (2006) Different predation impacts of two cyclopoid species on a small-sized zooplankton community: an experimental analysis with mesocosms. Hydrobiologia 556:233–242
Pastorok RA (1981) Prey vulnerability and size selection by Chaoborus larvae. Ecology 62:1311–1324
Rao TR, Kumar R (2002) Patterns of prey selectivity in cyclopoid copepod Mesocyclops thermocyclopoides. Aquat Ecol 36:411–424
Roche K (1990) Some aspects of vulnerability to cyclopoid predation of zooplankton prey individuals. Hydrobiologia 198:153–162
Sakamoto M, Chang KH, Hanazato T (2006) Inhibition of development of anti-predator morphology in the small cladoceran Bosmina by an insecticide: impact of an anthropogenic chemical on prey–predator interactions. Freshw Biol 51:1974–1983
Sakamoto M, Chang KH, Hanazato T (2007) Plastic phenotypes of antennule shape in Bosmina longirostris controlled by the physical stimuli from predators. Limnol Oceanogr 52:2072–2078
Santer B (1993) Do cyclopoid copepods control Daphnia populations in early spring, thereby protecting their juvenile instar stages from food limitation? Verh Int Verein Limnol 25:634–637
Tollrian R, Dodson SI (1999) Inducible defenses in Cladocera: constrains, costs, and multipredator environments. In: Tollrian R, Harvell CD (eds) The ecology and evolution of inducible defenses. Princeton University Press, New Jersey, pp 177–202
Williamson CE (1986) The swimming and feeding behavior of Mesocyclops. Hydrobiologia 134:11–19
Williamson CE (1993) Linking predation risk models with behavioral mechanisms: identifying population bottlenecks. Ecology 74:320–331
Yan ND, Girard R, Boudreau S (2002) An introduced invertebrate predator (Bythotrephes) reduces zooplankton species richness. Ecology 5:481–485
Acknowledgments
We thank Dr. T. Nagata and Dr. H. Takahashi for their helpful comments on our work. This study was partly supported by grants-in-aid to M. Sakamoto and T. Hanazato (F1910361) from a JSPS Research Fellowship for Young Scientists and grants-in-aid to T. Hanazato (no. 17201012) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakamoto, M., Hanazato, T. Antennule shape and body size of Bosmina: key factors determining its vulnerability to predacious Copepoda. Limnology 9, 27–34 (2008). https://doi.org/10.1007/s10201-007-0231-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-007-0231-3