Abstract
Background
Bone metastases are highly prevalent in breast, prostate, lung and colon cancers. Their symptoms negatively affect quality of life and functionality and optimal management can mitigate these problems. There are two different targeted agents to treat them: bisphosphonates (pamidronate and zoledronic acid) and the monoclonal antibody denosumab. Estimates of cost-effectiveness are still mixed.
Objective
To conduct a systematic review of economic studies that compares these two options.
Method
Literature search comprised eight databases and keywords for bone metastases, bisphosphonates, denosumab, and economic studies were used. Data were extracted regarding their methodologic characteristics and cost-effectiveness analyses. All studies were evaluated regarding to its methodological quality.
Results
A total of 263 unique studies were retrieved and six met inclusion criteria. All studies were based on clinical trials and other existing literature data, and they had high methodological quality. Most found unfavorable cost-effectiveness for denosumab compared with zoledronic acid, with adjusted ICERS that ranged from $4638–87,354 per SRE avoided and from US$57,274–4.81 M. per QALY gained, which varied widely according to type of tumor, time horizon, among others. Results were sensitive to drug costs, time to first skeletal-related event (SRE), time horizon, and utility.
Conclusions
Denosumab had unfavorable cost-effectiveness compared with zoledronic acid in most of the included studies. New economic studies based on real-world data and longer time horizons comparing these therapeutic options are needed.

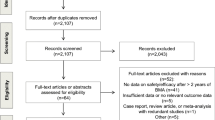
(adapted from the PRISMA statement)
Similar content being viewed by others
References
Aapro, M., Saad, F., Costa, L.: Optimizing clinical benefits of bisphosphonates in cancer patients with bone metastases. Oncologist 15, 1147–1158 (2010)
Machado, M., Cruz, L.S., Tannus, G., Fonseca, M.: Efficacy of clodronate, pamidronate, and zoledronate in reducing morbidity and mortality in cancer patients with bone metastasis: a meta-analysis of randomized clinical trials. Clin. Ther. 31(5), 962–979 (2009)
Espinosa, J.C., Baamonde, A.G.A., Herrero, F.R., Martín, E.H.: SEOM guidelines for the treatment of bone metastases from solid tumours. Clin. Transl. Oncol. 14, 505–511 (2012)
Choudhury, K.B., Mallik, C., Sharma, S., Choudhury, D.B., Maiti, S., Roy, C.: A randomized conrolled trial to compare the efficacy of bisphosphonates in the management of painful bone metastasis. Indian J. Palliat. Care 17(3), 210–218 (2011)
Mundy, G.R.: Metastasis to bone: causes, consequences and therapeutic opportunities. Nature 2(8), 584–593 (2002)
Qian, Y., Song, X., Zhang, K., Balakumaran, A., Arellano, J.: Short-term disability in solid tumor patients with bone metastases and skeletal-related events. J. Med. Econ. 18(3), 210–218 (2015)
Husaini, H.A., Wheatley-Price, P., Clemons, M., Shepherd, F.A.: Prevention and management of bone metastases in lung cancer: a review. J. Thorac. Oncol. 4(2), 251–259 (2009)
Zustovich, F., Fabiani, F.: Therapeutic opportunities for castration-resistant prostate cancer patients with bone metastases. Crit. Rev. Oncol. Hematol. 91, 197–209 (2014)
Coleman, R.E.: Risks and benefits of bisphosphonates. Br. J. Cancer 98, 1736–1740 (2008)
Carter, J.A., Joshi, A.D., Kaura, S., Botteman, M.F.: Pharmacoeconomics of bisphosphonates for skeletal related event prevention in metastatic non-breast solid tumours. Pharmacoeconomics 30(5), 373–386 (2012)
Food and Drug Administration (FDA): Highlights of prescribing information: zometa (zoledronic acid). http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021223s028lbl.pdf (2014). Accessed 25 Jul 2016
Coleman, R., Body, J.J., Aapro, M., Hadjii, P., Herretedt, J.: Bone health in cancer patients: ESMO clinical practice guidelines. Ann. Oncol. 25, 124–137 (2014)
Steger, G.G., Bartsch, R.: Denosumab for the treatment of bone metastasis in breast cancer: evidence and opinion. Ther. Adv. Med. Oncol. 3(5), 233–243 (2011)
Food and Drug Administration (FDA): Highlights of prescribing information: xgeva (denosumab). https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125320s094lbl.pdf (2013). Accessed 05 Jul 2017
Guimarães, H.P., Barbosa, L.M., Laranjeira, L.N., Avezum, A.: Pharmacoeconomic evaluations and economical analyses: basic concepts. Rev. Bras. Hipertens. 14(4), 265–268 (2007)
Secoli, S.R., Padilha, K.G., Litvoc, J., Maeda, S.T.: Pharmacoeconomics: resultant perspective of decisions process. Cien. Saude. Colet. 10, 287–296 (2005)
Goodwin, P.J.: Economic factors in cancer palliation—methodological considerations. Cancer Treat. Rev. 19, 59–65 (1993)
Bruner, D.W.: Cost-effectiveness and palliative care. Semin. Oncol. Nurs. 14(2), 164–167 (1998)
Greenberg, D., Earle, C., Fang, C., Eldar-Lissai, A., Neumann, P.J.: When is cancer care cost-effective? A systematic overview of cost-utility analysis in oncology. J. Natl. Cancer Inst. 102(2), 82–88 (2010)
Santos, C.M.C., Pimenta, C.A.M., Nobre, M.R.C.: A estratégia PICO para construção da pergunta de pesquisa e busca de evidências. Rev. Latino-am. Enfermagem. 15(3), 508–511 (2007)
Moher, D., Liberati, A., Tetzlaff, J., Altman, D.G.: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151(4), 264–269 (2009)
Sanders, G.D., Neumann, P.J., Basu, A., Brock, D.W., Feeny, D., Krahn, M., et al.: Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 316(10), 1093–1103 (2016)
Joanna Briggs Institute: The Joanna Briggs Institute critical appraisal tools for use in JBI systematic reviews: checklist for economic evaluations. http://joannabriggs.org/research/critical-appraisal-tools.html (2016). Accessed 24 Jul 2017
Phillips, B., Ball, C., Sackett, D., et al.: Oxford centre for evidence-based medicine levels of evidence grades of recommendation. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009 (2009). Accessed 24 Jul 2017
Arellano, J., Cristino, J., Chen, K.: Economic impact of denosumab for skeletal related event prevention in patients with prostate cancer and bone metastasis from a United States managed care organization perspective. Value Health 16(7), A398–A399 (2013)
Chen, K., Arellano, J., Cristino, J.: Economic impact of denosumab for skeletal related event prevention in patients with breast cancer and bone metastasis from a United States managed care organization perspective. Value Health 16(7), A400 (2013)
Rader, M.E., Danese, M., Conz, Z., Haplerin, M., Qian, Y., Goessl, C.D.: Lifetime cost effectiveness of denosumab versus zoledronic acid for prevention of skeletal-related events (SRE) in patients (pts) with castration-resistant prostate cancer (CRPC) and bone metastases (BM): United States managed care perspective. J. Clin. Oncol. 30(15), Suppl. 1 (2012)
Rader, M.E., Danese, M., Con, Z., Halperin, M., Qian, Y., Goessl, C.D., et al.: Cost-effectiveness of denosumab (Dmab) versus zoledronic acid (ZA) for the prevention of skeletal-related events (SREs) in patients (pts) with castrate-resistant prostate cancer (CRPC) and bone metastases (BM). J. Clin. Oncol. 30(5), Suppl. 1 (2012)
Chung, K., Rader, M.E., Danese, M., Cong, Z., Halperin, M., Qian, Y., et al.: Cost-effectiveness of denosumab versus zoledronic acid in the prevention of skeletal-related events (SREs) in patients with castration-resistant prostate cancer (CRPC) and bone metastases (BM). J. Oncol. Pharm. Pract. 18(Suppl1), 7–8 (2012)
Chung, K., Stopeck, A., Danese, M., Cong, Z., Halperin, M., Qian, Y., et al.: Cost-effectiveness of denosumab versus zoledronic acid in the prevention of skeletal-related events (SREs) in patients with breast cancer and bone metastases (BM). J. Oncol. Pharm. Pract. 18(Suppl1), 6–7 (2012)
Northridge, K., Richhariya, K., Halperin, M., Ghung, K., Danese, M.D.: Budget impact model of denosumab for skeletal-related event (SRE) prevention in patients with breast and prostate cancer. Value Health 4(3), A159 (2011)
Bell, M.J., Miller, J.D., Namjoshi, M., Russel, M.W.: Comparative budget impact of formulary inclusion of zoledronic acid and denosumab for prevention of skeletal-related events in patients with bone metastases. Value Health 4(3), A153 (2011)
Yu, A.P., Namjoshi, M.P., Xie, J., Parikh, K., Wu, E.Q., Guo, A., et al.: Economic evaluation of denosumab compared with zoledronic acid in patients with hormone refractory prostate cancer with bone metastases. J. Clin. Oncol. 29(15), Suppl. 1 (2011)
Russell, M.W., Bell, M.J., Namjoshi, M., Miller, J.D.: Financial impact of coverage for zoledronic acid and denosumab for prevention of skeletal-related events in cancer patients with bone metastases. J Clin Oncol. 29(15), Suppl. 1 (2011)
Arocho, R., Rivera Hurtado, R., Carlos, F.: Economic evaluation of denosumab versus zoledronic acid (ZA) in the prevention of skeletal-related events (SRE) in patients with prostate cancer with bone metastasis (BM) in Mexico. Value Health 16(3), A139–A140 (2013)
Alva, M.E., Naranjo, M., Zamora, J.: Economic evaluation of denosumab in the prevention of SRE in patients with breast cancer in Mexico. Value Health 19(3), A151–A152 (2016)
Duran, I., Seguí, M.A., Isla, D., Oyagüez, I., Roldán, C., Casado, M.A.: Cost-effectiveness of denosumab versus zoledronic acid in patients with bone metastases from solid tumors in Spain. Eur. J. Cancer 49(Suppl2), A334 (2013)
Lothgren, M., Bracco, A., Lucius, B., Northridge, K., Halperin, M., Macarios, D., et al.: Cost-effectiveness of denosumab versus zoledronic acid (ZA) for the prevention of skeletal-related events (SRE) in patients with bone metastases from solid tumors in the Netherlands. Value Health 14(7), A455 (2011)
Bektur, C., Nurgozhin, T.: Cost-effectiveness of denosumab vs. brand or generic zoledronic acid in patients with breast cancer in Kazakhstan. Value Health 17(7), A773 (2014)
Koo, K., Lam, K., Mittmann, N., Konski, A., Dennis, K., Zeng, L.: Comparing cost-effectiveness analyses of denosumab versus zoledronic acid for the treatment of bone metastases. Support. Care Cancer 21(6), 1785–1791 (2013)
Carter, J.A., Botteman, M.F.: Health-economic review of zoledronic acid for the management of skeletal-related events in bone-metastatic prostate cancer. Exp. Rev. Pharmacoecon. Outcomes Res. 12(4), 425–437 (2012)
Dellis, A., Papatsoris, A.: Cost-effectiveness of denosumab as a bone protective agent for patients with castration resistant prostate cancer. Exp. Rev. Pharmacoecon. Outcomes. Res. 16(1), 5–10 (2016)
Ford, J., Cummins, E., Sharma, P., Elders, A., Stewart, F., Johnston, R., et al.: Systematic review of the clinical effectiveness and cost-effectiveness, and economic evaluation, of denosumab for the treatment of bone metastases from solid tumours. Heath Technol. Assess. 17(29), 1–386 (2013)
Xie, J., Namjoshi, M., Wu, E.Q., Parikh, K., Diener, M., Yu, A.P.: Economic evaluation of debosumab compared with zoledronic acid in hormone refractory prostate cancer patients with bone metastases. J. Manag. Care Pharm. 17(8), 621–634 (2011)
Xie, J., Diener, M., Sorg, R., Wu, E.Q., Namjoshi, M.: Cost-effectiveness of denosumab compared with zoledronic acid in patients with breast cancer and bone metastases. Clin. Breast Cancer 12(4), 247–258 (2012)
Snedecor, S.J., Carter, J.A., Kaura, S., Botteman, M.F.: Cost-effectiveness of denosumab versus zoledronic acid in the management of skeletal metastases secondary to breast cancer. Clin. Ther. 34(6), 1334–1349 (2012)
Stopeck, A., Rader, M., Henry, D., Danese, M., Halperin, M., Cong, Z., et al.: cost-effectiveness of denosumab versus zoledronic acid for prevention of skeletal-related events in patients with solid tumors and bone metastases in the united states. J. Med. Econ. 15(4), 712–723 (2012)
Snedecor, S.J., Carter, J.A., Kaura, S., Botteman, M.F.: Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a cost-effectiveness analysis. J. Med. Econ. 16(1), 19–29 (2013)
Yfantopoulos, J., Christopoulou, A., Chatzikou, M., Fishman, P., Chalzaras, A.: The importance of economic evaluation in healthcare decision making—a case of denosumab versus zoledronic acid from Greece. A third-payer perspective. Forum Clin. Oncol. 4(2), 25–31 (2013)
Haas, J.S., Moskowitz, E.J.: Health technology assessment in canada and the United States: the case of biologics. Biotechnol. Health 4(2), 47–51 (2007)
Menon, D., Stafinski, T.: Health technology assessment in Canada: 20 years strong? Value Health 12(2), S14–S19 (2009)
Novaes, M.H.D., Elias, F.T.S.: Use of health technology assessment in decision-making processes by the Brazilian Ministry of Health on the incorporation of technologies in the Brazilian Unified National Health System. Cad. Saude Pública 29, S7–S16 (2013)
Novaes, M.H.D., Soárez, P.C.: Health technology assessment (HTA) organizations: dimensions of the institutional and political framework. Cad. Saúde Pública 32, S1–S14 (2016)
Carter, J.A., Snedecor, S.J., Kaura, S.: Cost effectiveness of zoledronic acid (ZOL) versus denosumab (Dmab) in prevention of skeletal related events (SREs) in metastatic breast cancer (mBC). J. Clin. Oncol. 29(suppl), abstract 9025 (2011)
Henry, D., Vadhan-Raj, S., Hirsh, V., von Moos, R., Hungria, V., Costa, C.: Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support. Care Cancer 22, 679–687 (2014)
Andronis, L., Goranitis, I., Bayliss, S., Duarte, R.: Cost-effectiveness of treatments for the management of bone metastases: a systematic literature review. PharmacoEconomics 36, 301–322 (2018)
Garattini, L., van de Vooren, K.: Budget impact analysis in economic evaluation: a proposal for a clearer definition. Eur. J. Health Econ. 12, 499–502 (2011)
Lexchin, J., Bero, L.A., Djulbegovic, B., Clark, O.: Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 326, 1167–1170 (2003)
Schott, G., Pachl, H., Limbach, U., Gundert-Remy, U., Ludwig, W.D., Lieb, K.: The financing of drug trials by pharmaceutical companies and its consequences. Dtsch. Arztebl. Int. 107(16), 279–285 (2010)
Neumann, P.J., Cohen, J.T., Weinstein, P.C.: Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N. Engl. J. Med. 371(9), 796–797 (2014)
Rascati, K.L.: Criticando Artigos de Pesquisa. In: Rascati, K.L. (ed.) Introducão à Farmacoeconomia, pp. 45–54. Artmed, Porto Alegre (2010)
Cohen, D.J., Reynolds, M.R.: Interpreting the results of cost-effectiveness studies. J. Am. Coll. Cardiol. 52(25), 2119–2126 (2008)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human/animal rights statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Matuoka, J.Y., Kahn, J.G. & Secoli, S.R. Denosumab versus bisphosphonates for the treatment of bone metastases from solid tumors: a systematic review. Eur J Health Econ 20, 487–499 (2019). https://doi.org/10.1007/s10198-018-1011-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-018-1011-1