Abstract
Background
Individuals who have kidney disease or kidney transplants need routine assessment of their kidney damage and function, which are largely measured based on histological examination of kidney biopsies, blood test, and urinalysis. These methods are practically difficult or inconvenient, and expensive. The objective of this study was to develop a model to estimate the kidney damage and function by surface-enhanced Raman spectroscopy (SERS).
Methods
Urine samples were collected from two previous studies: renal allograft recipient Lewis rats receiving anti-TGF-β antibody or control antibody treatment and obese diabetic ZSF1 rats with kidney disease fed with whole grape powder-containing chow or control chow. Silver nanoparticle-based SERS spectra of urine were measured. SERS spectra were analyzed using principal component analysis (PCA) combined with linear discriminant analysis (LDA) and partial least squires (PLS) analysis.
Results
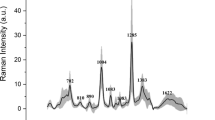
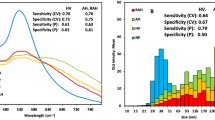
PCA/LDA separated anti-TGF-β antibody-treated group from control group with 90% sensitivity and 70% specificity in kidney transplants, and grape-fed group from controls with 72.7% sensitivity and 60% specificity in diabetic kidneys. The receiver operating characteristic curves showed that the integration area under the curve was 0.850 ± 0.095 (p = 0.008) in kidney transplant groups and 0.800 ± 0.097 (p = 0.02) in diabetic kidney groups. PLS predicted the biochemical parameters of kidney function using the SERS spectra, resulting in R2 = 0.8246 (p < 0.001,urine protein), R2 = 0.8438 (p < 0.001, urine creatinine), R2 = 0.9265 (p < 0.001, urea), R2 = 0.8719 (p < 0.001, serum creatinine), and R2 = 0.6014 (p < 0.001, urine protein to creatinine ratio).
Conclusion
Urine SERS spectral analysis suggesting that it may become a convenient method for rapid assessment of renal impairment.




Similar content being viewed by others
References
Levin A, Hemmelgarn B, Culleton B, Tobe S, McFarlane P, Ruzicka M, et al. Guidelines for the management of chronic kidney disease. CMAJ Can Med Assoc J. 2008;179(11):1154–62. https://doi.org/10.1503/cmaj.080351.
Josephson MA. Monitoring and managing graft health in the kidney transplant recipient. Clin J Am Soc Nephrol CJASN. 2011;6(7):1774–80. https://doi.org/10.2215/CJN.01230211.
Vassalotti JA, Centor R, Turner BJ, Greer RC, Choi M, Sequist TD, et al. Practical approach to detection and management of chronic kidney disease for the primary care clinician. Am J Med. 2016;129(2):153 e7–62 e7. https://doi.org/10.1016/j.amjmed.2015.08.025.
Wouters OJ, O’Donoghue DJ, Ritchie J, Kanavos PG, Narva AS. Early chronic kidney disease: diagnosis, management and models of care. Nat Rev Nephrol. 2015;11(8):491–502. https://doi.org/10.1038/nrneph.2015.85.
Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473–83. https://doi.org/10.1056/NEJMra054415.
Bauer C, Melamed ML, Hostetter TH. Staging of chronic kidney disease: time for a course correction. J Am Soc Nephrol JASN. 2008;19(5):844–6. https://doi.org/10.1681/ASN.2008010110.
Lin J, Knight EL, Hogan ML, Singh AK. A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol JASN. 2003;14(10):2573–80.
Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141(12):929–37. https://doi.org/10.7326/0003-4819-141-12-200412210-00009.
Rule AD, Gussak HM, Pond GR, Bergstralh EJ, Stegall MD, Cosio FG, et al. Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis. 2004;43(1):112–9.
Poggio ED, Wang X, Greene T, Van Lente F, Hall PM. Performance of the modification of diet in renal disease and Cockcroft–Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol JASN. 2005;16(2):459–66. https://doi.org/10.1681/ASN.2004060447.
Stevens LA, Manzi J, Levey AS, Chen J, Deysher AE, Greene T, et al. Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am J Kidney Dis. 2007;50(1):21–35. https://doi.org/10.1053/j.ajkd.2007.04.004.
Stevens LA, Nolin TD, Richardson MM, Feldman HI, Lewis JB, Rodby R, et al. Comparison of drug dosing recommendations based on measured GFR and kidney function estimating equations. Am J Kidney Dis. 2009;54(1):33–42. https://doi.org/10.1053/j.ajkd.2009.03.008.
Murata K, Baumann NA, Saenger AK, Larson TS, Rule AD, Lieske JC. Relative performance of the MDRD and CKD-EPI equations for estimating glomerular filtration rate among patients with varied clinical presentations. Clin J Am Soc Nephrol. 2011;6(8):1963–72. https://doi.org/10.2215/Cjn.02300311.
Yilmaz S, Isik I, Afrouzian M, Monroy M, Sar A, Benediktsson H, et al. Evaluating the accuracy of functional biomarkers for detecting histological changes in chronic allograft nephropathy. Transpl Int. 2007;20(7):608–15. https://doi.org/10.1111/j.1432-2277.2007.00494.x.
Schrader B. Chemical applications of raman spectroscopy. Angew Chem Int Ed. 1973;12(11):884–908. doi:https://doi.org/10.1002/anie.197308841.
Baena JR, Lendl B. Raman spectroscopy in chemical bioanalysis. Curr Opin Chem Biol. 2004;8(5):534–9. https://doi.org/10.1016/j.cbpa.2004.08.014.
Dumont E, De Bleye C, Sacre PY, Netchacovitch L, Hubert P, Ziemons E. From near-infrared and Raman to surface-enhanced Raman spectroscopy: progress, limitations and perspectives in bioanalysis. Bioanalysis. 2016;8(10):1077–103. https://doi.org/10.4155/bio-2015-0030.
Hudson SD, Chumanov G. Bioanalytical applications of SERS (surface-enhanced Raman spectroscopy). Anal Bioanal Chem. 2009;394(3):679–86. https://doi.org/10.1007/s00216-009-2756-2.
Feng S, Chen R, Lin J, Pan J, Chen G, Li Y, et al. Nasopharyngeal cancer detection based on blood plasma surface-enhanced Raman spectroscopy and multivariate analysis. Biosens Bioelectron. 2010;25(11):2414–9. https://doi.org/10.1016/j.bios.2010.03.033.
Badr Y, Mahmoud MA. Effect of silver nanowires on the surface-enhanced Raman spectra (SERS) of the RNA bases. Spectrochim Acta Part A Mol Biomol Spectrosc. 2006;63(3):639–45. https://doi.org/10.1016/j.saa.2005.06.013.
Han XX, Zhao B, Ozaki Y. Surface-enhanced Raman scattering for protein detection. Anal Bioanal Chem. 2009;394(7):1719–27. https://doi.org/10.1007/s00216-009-2702-3.
Harper MM, Dougan JA, Shand NC, Graham D, Faulds K. Detection of SERS active labelled DNA based on surface affinity to silver nanoparticles. Analyst. 2012;137(9):2063–8. https://doi.org/10.1039/c2an35112a.
Wang H, Malvadkar N, Koytek S, Bylander J, Reeves WB, Demirel MC. Quantitative analysis of creatinine in urine by metalized nanostructured parylene. J Biomed Opt. 2010;15(2):027004. https://doi.org/10.1117/1.3369002.
Bispo JA, de Sousa Vieira EE, Silveira L Jr, Fernandes AB. Correlating the amount of urea, creatinine, and glucose in urine from patients with diabetes mellitus and hypertension with the risk of developing renal lesions by means of Raman spectroscopy and principal component analysis. J Biomed Opt. 2013;18(8):87004. https://doi.org/10.1117/1.JBO.18.8.087004.
Chi JM, Zaw T, Cardona I, Hosnain M, Garg N, Lefkowitz HR, et al. Use of surface-enhanced Raman scattering as a prognostic indicator of acute kidney transplant rejection. Biomed Opt Express. 2015;6(3):761–9. https://doi.org/10.1364/Boe.6.000761.
Huang SH, Wang L, Chen WS, Feng SY, Lin JQ, Huang ZF, et al. Potential of non-invasive esophagus cancer detection based on urine surface-enhanced Raman spectroscopy. Laser Phys Lett. 2014;11(11). https://doi.org/10.1088/1612-2011/11/11/115604.
Lin X, Lin D, Ge X, Qiu S, Feng S, Chen R. Noninvasive detection of nasopharyngeal carcinoma based on saliva proteins using surface-enhanced Raman spectroscopy. J Biomed Opt. 2017;22(10):1–6. https://doi.org/10.1117/1.JBO.22.10.105004.
Feng S, Lin D, Lin J, Li B, Huang Z, Chen G, et al. Blood plasma surface-enhanced Raman spectroscopy for non-invasive optical detection of cervical cancer. Analyst. 2013;138(14):3967–74. https://doi.org/10.1039/c3an36890d.
Feng S, Chen R, Lin J, Pan J, Wu Y, Li Y, et al. Gastric cancer detection based on blood plasma surface-enhanced Raman spectroscopy excited by polarized laser light. Biosens Bioelectron. 2011;26(7):3167–74. https://doi.org/10.1016/j.bios.2010.12.020.
Lin D, Feng S, Pan J, Chen Y, Lin J, Chen G, et al. Colorectal cancer detection by gold nanoparticle based surface-enhanced Raman spectroscopy of blood serum and statistical analysis. Opt Express. 2011;19(14):13565–77. https://doi.org/10.1364/OE.19.013565.
Li SX, Zeng QY, Li LF, Zhang YJ, Wan MM, Liu ZM, et al. Study of support vector machine and serum surface-enhanced Raman spectroscopy for noninvasive esophageal cancer detection. J Biomed Opt. 2013;18(2):27008. https://doi.org/10.1117/1.JBO.18.2.027008.
Bonifacio A, Dalla Marta S, Spizzo R, Cervo S, Steffan A, Colombatti A, et al. Surface-enhanced Raman spectroscopy of blood plasma and serum using Ag and Au nanoparticles: a systematic study. Anal Bioanal Chem. 2014;406(9–10):2355–65. https://doi.org/10.1007/s00216-014-7622-1.
Feng S, Zheng Z, Xu Y, Lin J, Chen G, Weng C, et al. A noninvasive cancer detection strategy based on gold nanoparticle surface-enhanced raman spectroscopy of urinary modified nucleosides isolated by affinity chromatography. Biosens Bioelectron. 2017;91:616–22. https://doi.org/10.1016/j.bios.2017.01.006.
Mukanova Z, Gudun K, Elemessova Z, Khamkhash L, Ralchenko E, Bukasov R. Detection of paracetamol in water and urea in artificial urine with gold nanoparticle@Al foil cost-efficient SERS substrate. Anal Sci Int J Jpn Soc Anal Chem. 2018;34(2):183–7. https://doi.org/10.2116/analsci.34.183.
Guan Q, Li S, Gao S, Chen H, Nguan CY, Du C. Reduction of chronic rejection of renal allografts by anti-transforming growth factor-beta antibody therapy in a rat model. Am J Physiol Ren Physiol. 2013;305(2):F199–207. https://doi.org/10.1152/ajprenal.00665.2012.
Almomen SM, Guan Q, Liang P, Yang K, Sidiqi AM, Levin A, et al. Daily intake of grape powder prevents the progression of kidney disease in obese type 2 diabetic ZSF1 rats. Nutrients. 2017. https://doi.org/10.3390/nu9040345.
Leopold N, Lendl B. A new method for fast preparation of highly surface-enhanced Raman scattering (SERS) active silver colloids at room temperature by reduction of silver nitrate with hydroxylamine hydrochloride. J Phys Chem B. 2003;107(24):5723–7. https://doi.org/10.1021/jp027460u.
Huang Z, Zeng H, Hamzavi I, McLean DI, Lui H. Rapid near-infrared Raman spectroscopy system for real-time in vivo skin measurements. Opt Lett. 2001;26(22):1782–4.
Zhao JH, Lui H, McLean DI, Zeng HS. Integrated real-time Raman system for clinical in vivo skin analysis. Skin Res Technol. 2008;14(4):484–92. https://doi.org/10.1111/j.1600-0846.2008.00321.x.
Zhao J, Lui H, McLean DI, Zeng H. Automated autofluorescence background subtraction algorithm for biomedical Raman spectroscopy. Appl Spectrosc. 2007;61(11):1225–32. https://doi.org/10.1366/000370207782597003.
Saatkamp CJ, de Almeida ML, Bispo JA, Pinheiro AL, Fernandes AB, Silveira L Jr. Quantifying creatinine and urea in human urine through Raman spectroscopy aiming at diagnosis of kidney disease. J Biomed Opt. 2016;21(3):37001. https://doi.org/10.1117/1.JBO.21.3.037001.
Zhao JH, Zeng HS, Kalia S, Lui H. Using Raman spectroscopy to detect and diagnose skin cancer in vivo. Dermatol Clin. 2017;35(4):495. https://doi.org/10.1016/j.det.2017.06.010.
Lin D, Qiu S, Huang W, Pan J, Xu Z, Chen R, et al. Autofluorescence and white light imaging guided endoscopic Raman and diffuse reflectance spectroscopy for in vivo nasopharyngeal cancer detection. J Biophoton. 2017. https://doi.org/10.1002/jbio.201700251.
Lin D, Huang H, Qiu S, Feng S, Chen G, Chen R. Diagnostic potential of polarized surface enhanced Raman spectroscopy technology for colorectal cancer detection. Opt Express. 2016;24(3):2222–34. https://doi.org/10.1364/OE.24.002222.
Li JT, Du Y, Qi J, Sneha R, Chang A, Mohan C, et al. Raman spectroscopy as a diagnostic tool for monitoring acute nephritis. J Biophoton. 2016;9(3):260–9. https://doi.org/10.1002/jbio.201500109.
Talari ACS, Movasaghi Z, Rehman S, Rehman IU. Raman spectroscopy of biological tissues. Appl Spectrosc Rev. 2015;50(1):46–111. https://doi.org/10.1080/05704928.2014.923902.
McMurdy JW III, Berger AJ. Raman spectroscopy-based creatinine measurement in urine samples from a multipatient population. Appl Spectrosc. 2003;57(5):522–5. https://doi.org/10.1366/000370203321666533.
Qi D, Berger AJ. Chemical concentration measurement in blood serum and urine samples using liquid-core optical fiber Raman spectroscopy. Appl Opt. 2007;46(10):1726–34.
Dou X, Yamaguchi Y, Yamamoto H, Doi S, Ozaki Y. Quantitative analysis of metabolites in urine using a highly precise, compact near-infrared Raman spectrometer. Vib Spectrosc. 1996;13(1):83–9. https://doi.org/10.1016/0924-2031(96)00036-7.
Wang TL, Chiang HK, Lu HH, Peng FY. Semi-quantitative surface enhanced Raman scattering spectroscopic creatinine measurement in human urine samples. Opt Quant Electron. 2005;37(13–15):1415–22. https://doi.org/10.1007/s11082-005-4221-6.
Li M, Du Y, Zhao F, Zeng J, Mohan C, Shih WC. Reagent- and separation-free measurements of urine creatinine concentration using stamping surface enhanced Raman scattering (S-SERS). Biomed Opt Express. 2015;6(3):849–58. https://doi.org/10.1364/BOE.6.000849.
Acknowledgements
This work is supported by China Scholarship Council and Michael Smith Foundation for Health Research of Canada.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicting financial interests.
Human and animal rights statement
All the animals used in the study were approved by and passed the ethical standards of University of British Columbia. All the human samples used in the study were approved by and passed the ethical standard of both the University of British Columbia and the West China Hospital, Sichuan University.
Informed consent
All patients filled the informed consents prior to the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Feng, S., Zhou, L., Lin, D. et al. Assessment of treatment efficacy using surface-enhanced Raman spectroscopy analysis of urine in rats with kidney transplantation or kidney disease. Clin Exp Nephrol 23, 880–889 (2019). https://doi.org/10.1007/s10157-019-01721-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-019-01721-w