Abstract
Background
Diabetes mellitus (DM) is a major cause of end-stage kidney disease (ESKD). However, the difference in renal outcomes between DM patients with non-diabetic renal disease (DM and NDRD) and those with diabetic nephropathy (DN) is controversial. The aim of the present study was to evaluate the differences among patients with DN, DM, and NDRD, and non-DM chronic kidney disease (CKD) in a prospective observational study.
Methods
We extracted the data of 2484 patients from 11 nephrology care centers and categorized into three groups as described above. The primary outcome was ESKD requiring renal replacement therapy.
Results
During the median follow-up of 4.44 years, 281 patients (11.3%) developed ESKD. Renal outcomes of DM and NDRD patients were similar to those of non-DM patients (p ≥ 0.05). At CKD stage G3b, the hazard ratios (95% confidence intervals) of ESKD were 7.10 (2.46–20.49) in DN patients and 0.89 (0.19–4.24) in DM and NDRD. The annual change in the estimated glomerular filtration rate (eGFR) in DN patients was significantly larger than that in other groups at stage G3b (−9.7%/year).
Conclusions
We found that DN patients have a higher risk for ESKD than DM and NDRD or non-DM patients. In particular, GFR rapidly declined in DN at stage G3b. DM and NDRD patients can accomplish equally beneficial renal outcomes as non-DM CKD, regardless of their similar metabolic profiles as DN. In conclusion, we should prudentially consider the risk stratification of DM whether cause or comorbidity of CKD.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is a serious public health issue worldwide because of the increasing cases with end-stage kidney disease (ESKD) [1] and risk of cardiovascular disease [2]. One of the major causes of ESKD is diabetic kidney disease (DKD), which is a syndrome that affects the kidneys of patients with diabetes; thus, DKD is one of the interventional targets to avoid the progression of CKD [3]. In Japan, the number of patients requiring dialysis exceeds 2500/million which is second highest in the world [4]. Diabetic nephropathy (DN) accounts for 40% of all cases of ESKD. The classical definition of DN is CKD due to diabetes mellitus (DM) [5]. Conversely, DM patients with non-DM-mediated CKD complications [DM and non-diabetic renal disease (NDRD)] are broadly included within DKD. Nevertheless, only a few reports have compared the clinical characteristics and renal outcomes between DN vs. DM and NDRD [6, 7]. Furthermore, the impact of DM as a complication of kidney disease is controversial in nephrology care because of various risk factors in addition to DM [8, 9].
We previously reported that DN patients have a poor renal prognosis compared to those with other causes of CKD, using univariate analysis [10]. However, there were several limitations in the previous study regarding relatively short observation periods (12 months) and the baseline of lower estimated glomerular filtration rate (eGFR) in DN patients [10]. Hence, longer observation periods and the use of stratified analyses by baseline CKD stage have been suggested to improve our understanding of these conditions.
Therefore, the present study aimed to determine the differences among DN, DM, and NDRD, and non-DM patients by comparing the clinical characteristics and 5-year renal outcomes within each CKD stage.
Materials and methods
Study design
The present investigation was a multi-center prospective observational cohort study. The study included 10 hospitals and 1 clinic that provide nephrology care in Miyagi prefecture located in the northeast region of Japan, which has a population of 2.3 million.
Setting and participants’ inclusion criteria
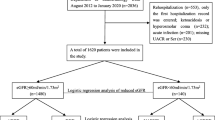
A flowchart representing the criteria for patients’ inclusion into the present study is shown in Fig. 1. Outpatients aged ≥20 years who were receiving nephrology care were considered for inclusion. The study initially included 4015 patients who received care between May 2006 and November 2008, and maintained their annual scheduled follow-up. CKD was defined as eGFR of <60 mL/min/1.73 m2, urinary albumin excretion continuing for >3 months, or the diagnosis of kidney disease by imaging or histological analysis. The study excluded 888 patients because of their inconsistencies with the CKD definition [11]. Among the rest, patients were excluded if they lacked laboratory data or medical history essential for this analysis. Accordingly, the risk of ESKD was finally analyzed in 2484 patients.
The data of 135 patients, who were affected with ESKD and lacked eGFR data at the end of the follow-up period, were interpolated using the mean eGFR (6.33 mL/min/1.73 m2) of patients who showed ESKD emergence. Finally, the annual change in eGFR was analyzed in 1844 patients after excluding 388 patients who lacked follow-up eGFR data, 219 patients who had been observed for less than 1 year, and 33 extreme deviations (≥3 × standard deviation) from the mean eGFR decline rate.
Classification of patients
Patients were classified into three groups as (1) DN (n = 249), (2) DM and NDRD (n = 448), and (3) non-DM (n = 1787) according to the underlying renal diseases as previously described [10]. DM was considered when the patients’ medical record showed DM, or if the patient had fasting blood glucose levels ≥126 mg/dL or non-fasting glucose levels >200 mg/dL, or if they were undergoing diabetes treatment. The distinction between DN vs. DM and NDRD was made based on renal histopathological or clinical diagnoses. Diagnoses in 47.5% of the DM and NDRD patients and 18.1% of the DN patients were validated by a kidney biopsy. The rationale classified as DM and NDRD was summarized in Table 1.
A substantial number of patients were diagnosed as DN during diabetes care and were referred to nephrology care with diabetic retinopathy or neuropathy, including those with insufficient long-term (approximately over 5 years) control of DM.
The stages of CKD are classified from stage G1 to G5 on the basis of eGFR [11]: G1, 2: eGFR >60 mL/min/1.73 m2; G3a: eGFR 45–59 mL/min/1.73 m2; G3b: eGFR 30–44 mL/min/1.73 m2: G4, eGFR 15–29 mL/min/1.73 m2; and G5: eGFR <15 mL/min/1.73 m2. Nephrologists who conducted the diagnosis for each subgroup at the time of registration were not involved in any data analysis in this study.
Follow-up and outcomes
Registration was conducted from 2006 to 2008, and patients were followed up by 2011–2013. The primary outcome was ESKD during the observation period of 5 years. ESKD was defined as the commencement of dialysis in our hospital, requirement for dialysis by other hospitals, and/or kidney transplantation. Patients were censored at death from all causes.
We evaluated the annual eGFR changes (%) among the three groups using the following formula: 100 × (eGFR at the end of follow-up − baseline eGFR)/(baseline eGFR)/(observation days/365.25) [12]. In this analysis, we used data obtained by the end of March 2011.
Measurements and data collection
Patient data were obtained annually from the medical records at each hospital. Blood pressure were measured in the sitting position, using an automatic sphygmomanometer based on the Korotkoff sound technique at outpatient clinics affiliated with the local medical centers. We collected the clinical laboratory data of urine and blood samples measured at each participating hospital. Serum creatinine levels were measured using the enzyme assay method in all laboratories.
Cardiac disease included heart failure, angina, and myocardial infarction. Heart failure was defined as the need for all general admission. Angina and myocardial infarction were diagnosed based on the findings of imaging. Patients receiving lipid-lowering drugs and/or those with serum total cholesterol levels >220 mg/dL were considered to have hypercholesterolemia.
Statistical analysis
Comparisons of baseline characteristics between DN, DM, and NDRD, and non-DM groups were examined using χ 2 test (for categorical variables) or analysis of variance (for continuous variables). Survival analyses for progression to ESKD were determined using the log-rank test. Associations between ESKD events and underlying diseases were examined using Cox hazard model analysis adjusted for age, sex, smoking status, body mass index, systolic blood pressure, history of cardiovascular disease or dyslipidemia, use of anti-hypertensive or lipid-lowering drugs, presence of hematuria and proteinuria, and eGFR. Annual changes in eGFR were calculated using analysis of covariance adjusted for the above-mentioned covariates and the follow-up time.
Data are presented as mean ± standard deviation. A p value <0.05 indicated statistical significance. All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
The number of patients with DM was 697 (28.1%) among the 2484 CKD patients. The rate of DM patients was similar to that of other CKD cohorts [13, 14]. According to the grouping criteria of kidney damage, 249 (10.0%) were classified as DN, and the remaining 448 (18.1%) were classified as DM and NDRD. The median observation period was 4.44 years (0.11–5.08 years).
Baseline patient characteristics are shown in Table 2. Increased age, elevated body mass index, increased systolic blood pressure, high proportion of males, and patients with dyslipidemia were more common in the DN and DM and NDRD groups than in the non-DM group. Furthermore, systolic blood pressure was higher in the DN group than in the DM and NDRD group. The smoking status, serum creatinine levels, proteinuria, and cardiac disease status were significantly higher in the DN group than in the DM and NDRD and non-DM groups. The baseline CKD stages were distinctive in DN, i.e., stage G4 or G5 accounted for 128 patients (51.4%) at the time of enrollment. With respect to the origin of kidney disease, hypertensive nephropathy was more common in the DM and NDRD group than in the non-DM group. There was a significant difference in primary disease comparing except “diabetic nephropathy” and DN groups (p < 0.0001).
Glycosylated hemoglobin (HbA1c) data were obtained in 624 out of 697 subjects who had DM at the time of enrollment. Difference in HbA1c values was observed only in CKD stage G1, 2 between DM and DNRD (7.1 ± 1.4%) and DN (7.7 ± 1.6%) patients (p = 0.01, Table 3). The prevalence of high HbA1c (>7%) was 37.3% in DM and NDRD and 37.9% in DN (p = 0.9). No significant differences were observed in the analysis of renal outcome.
During the median follow-up period of 4.44 years, 281 patients (11.3%) developed ESKD. The survival curves of patients at CKD stages G3b, G4, and G5 are shown in Fig. 2. The DN group showed significantly higher progression to ESKD not only stratified by the stage of CKD, but also by overall analysis (p < 0.0001). At CKD stages G1, 2, and G3a, the number of ESKD events was limited (only 7 among 1549) during observation period.
When the non-DM group was used as the reference group at each CKD stage, the adjusted hazard ratio of ESKD was significantly higher in DN patients than in non-DM patients with CKD stages G3b, G4, and G5 (Table 4). However, there was no significant difference between the DM and NDRD and non-DM groups. Moreover, patients with DM and NDRD also showed a lower risk of ESKD than DN group at CKD stage G3b (p = 0.01) and G4 (p = 0.06, Table 4).
Annual decline of eGFR among the three groups was compared in 1844 patients who had more than 1 year of follow-up eGFR data (Table 5). DN patients showed significantly faster decline of eGFR compared to the other groups at the stage of CKD G3b. However, in DN patients of negative proteinuria, only 2 patients of G5 and 1 patient of G4 developed to ESKD.
Discussion
There are 3 key novel findings observed in the present analysis of the Gonryo study. First, there were no significant differences between the DM and NDRD vs. non-DM CKD groups in terms of renal outcomes. Second, there was acceleration in the annual decline of eGFR in the DN group at stage G3b under nephrology care. Third, the DN group progressed to ESKD more rapidly than DM and NDRD or non-DM groups.
Hemmelgarn et al. have reported that the eGFR decline was the highest in subjects with DM than non-DM within the community-dwelling elderly [15]. However, in the report, the general population cohort was not divided into DN and DM and NDRD. Byun et al. have reported that DM and NDRD are associated with better renal outcomes than DN in renal biopsy reports of 110 patients with type 2 diabetes [6]. However, the study did not compare with non-DM patients. In the current disease-based cohort study, the characteristics of diabetes mellitus [16] such as age, male dominance, systolic blood pressure, and dyslipidemia were similar in the DN and DM and NDRD groups, even though eGFR of the DM and NDRD group could be maintained similar to that of the non-DM group. The frequency of high HbA1c (>7.0%) was comparable between DM and NDRD (37.3%) and DN (37.9%).
DM and NDRD group had a higher prevalence of hypertensive nephrosclerosis as the cause of kidney disease compared to the non-DM group (31.7 vs. 14.2%). Different outcomes of DM and NDRD and DN in our study may be attributed to the difference in primary disease. Furthermore, our result showed that nephrological intervention in DM and NDRD patients demonstrated favorable renal prognosis nearly equal to that in non-DM patients. These results support the recommendation of American Diabetes Association guideline that DKD patients should be referred to nephrologists promptly when there is uncertainty about the etiology of DKD [17].
At the late stages of CKD, burden of hyperfiltration is mainly ascribed to compensation of disrupted nephrons, rather than hyperglycemia. Hence, after CKD stage G3a, HbA1c management conditions of DM and NDRD were equal to that of DN and did not mediate the different renal outcome of them. By contrast, hyperfiltration arising from hyperglycemia plays a key role in renal impairment during early nephropathy [18, 19]. Our results showed a high HbA1c value in DN patients at CKD stage G1, 2, which may reflect that poorly controlled HbA1c at early CKD stages cause the rapid renal impairment after G3b. It is speculated that G3b is the point of breakdown in compensation of hyperfiltration arising from hyperglycemia at G1, 2. However, longer term observation time is required when evaluating renal outcomes of G1 and 2 patients. Thus, physicians should consider different contributions of hyperglycemia to renal dysfunction between early and late CKD stages.
This Gonryo cohort study is distinguished for classifying patients on the basis of kidney disease. The 2 years’ interim analysis that the proportion of patients enrolled with CKD stage G4 or G5 was larger in DN (51.3%) than in the DM and NDRD and non-DM (18.6%) groups [20]. Due to this baseline profile, it was feasible to evaluate the incidence of ESKD in DN. Consequently, we evaluated the renal outcomes according to the matrix of CKD stages at the time of enrollment, in addition to the relationship between CKD and diabetes.
As a benefit of stratification analysis by CKD stage, we found out that the initiation of rapid deterioration in eGFR levels occurred at CKD stage G3b in DN patients. Djamali et al. reported that the mean decline rate of creatinine clearance was 5.4 ml/min/year in stage 3 including G3a and G3b as one category [21]. On the contrary, our present study revealed the critical differences between the G3a and G3b stages, especially in DN patients. The rapid decline of eGFR at stage G3b in DN patients may have a strong correlation with proteinuria. The importance of proteinuria has been shown in the previous cohort studies [22,23,24]. Our results suggest that early referral and strategic intervention are necessary for stage G3a DN patients to avoid deterioration.
Our study has several limitations. First, this cohort may have selection bias. Even though the health care system has easy access to nephrology care like Japan, many DN patients face difficulties in receiving earlier referrals to prevent deterioration [25]. Exacerbation of proteinuria and decline of GFR were the basis of consultation or transition to nephrology clinic from diabetes clinics in DN. Second, DM and NDRD patients without renal biopsy were classified using medical history of NDRD. Serological marker like autoantibody, imaging study, and well controlled without retinopathy or neuropathy were implicated in the diagnosis of NDRD. Furthermore, the collaborate nephrology specialists differentiated between DN and DM and NDRD including the factor of DM duration, even though we did not included it in our case report form which the study design focused mainly kidney disease than diabetes. Nevertheless, recent study reported that the diabetic patients with CKD stage G1A1 sometimes reveal diabetic nodular lesions [26]. It is suggesting the difficulty of the diagnosis of DN without renal biopsy. Consequently, some patients of DN might be classified as DM and NDRD in this analysis. The other limitation exists in the evaluation of glycemic control in various stages of CKD due to the influence of renal anemia and hypoalbuminemia [27, 28]. The HbA1c levels were similar between the 2 diabetic groups at the time of enrollment and our data were relatively within fair ranges according to American Diabetes Association guidelines of kidney impaired patients [17]. Furthermore, a high incidence of ESKD was observed in low HbA1c because of correlation between HbA1c and eGFR. Consequently, high HbA1c levels were not the risk for progression in CKD stage G3b and above.
Conclusions
We found that DN patients have a higher risk for developing ESKD than DM and NDRD or non-DM patients. Optimal therapeutic intervention in DM and NDRD can accomplish equally beneficial renal outcomes as non-DM CKD, regardless of their similar metabolic profiles as DN patients. We focused G3b of DN that eGFR declined rapidly in nephrology care. It needs to make further investigation whether G3a stage period is extended or eGFR gradient in G3b is attenuated by nephrology intervention in DN [25]. We, however, should prudentially consider the risk stratification of DM whether cause or comorbidity of CKD.
References
Arogundade FA, Barsoum RS. CKD prevention in Sub-Saharan Africa: a call for governmental, nongovernmental, and community support. Am J Kidney Dis. 2008;51:515–23.
Drey N, Roderick P, Mullee M, Rogerson M. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis. 2003;42:677–84.
KDOQI. Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis 2012; 60:850-86.
Masakane I, Nakai S, Ogata S, Kimata N, Hanafusa N, Hamano T, et al. An overview of regular dialysis treatment in Japan (as of 31 December 2013). Ther Apher Dial. 2015;19:540–74.
Lim A. Diabetic nephropathy—complications and treatment. Int J Nephrol Renovasc Dis. 2014;7:361–81.
Byun JM, Lee CH, Lee SR, Moon JY, Lee SH, Lee TW, et al. Renal outcomes and clinical course of nondiabetic renal diseases in patients with type 2 diabetes. Korean J Intern Med. 2013;28:565–72.
Wong TY, Choi PC, Szeto CC, To KF, Tang NL, Chan AW, et al. Renal outcome in type 2 diabetic patients with or without coexisting nondiabetic nephropathies. Diabetes Care. 2002;25:900–5.
Chen HC, Chou CY, Hsiao YT, Liang CC, Kuo HL, Chang CT, et al. Patients with diabetes as the primary kidney disease have a worse survival than patients with comorbid diabetes in chronic haemodialysis patients. Nephrology (Carlton). 2015;20:155–60.
Lei Y, Xiong Y, Zhang L, Yan H, Li Z, Cao L, et al. Comparison of long-term outcomes between peritoneal dialysis patients with diabetes as a primary renal disease or as a comorbid condition. PLoS One. 2015;10:e0126549.
Nakayama M, Sato T, Sato H, Yamaguchi Y, Obara K, Kurihara I, et al. Different clinical outcomes for cardiovascular events and mortality in chronic kidney disease according to underlying renal disease: the Gonryo study. Clin Exp Nephrol. 2010;14:333–9.
KDIGO. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150.
Turin TC, Coresh J, Tonelli M, Stevens PE, de Jong PE, Farmer CK, et al. Short-term change in kidney function and risk of end-stage renal disease. Nephrol Dial Transplant. 2012;27:3835–43.
De Nicola L, Chiodini P, Zoccali C, Borrelli S, Cianciaruso B, Di Iorio B, et al. Prognosis of CKD patients receiving outpatient nephrology care in Italy. Clin J Am Soc Nephrol. 2011;6:2421–8.
Levin A, Djurdjev O, Barrett B, Burgess E, Carlisle E, Ethier J, et al. Cardiovascular disease in patients with chronic kidney disease: getting to the heart of the matter. Am J Kidney Dis. 2001;38:1398–407.
Hemmelgarn BR, Zhang J, Manns BJ, Tonelli M, Larsen E, Ghali WA, et al. Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int. 2006;69:2155–61.
Mukai N, Doi Y, Ninomiya T, Hata J, Yonemoto K, Iwase M, et al. Impact of metabolic syndrome compared with impaired fasting glucose on the development of type 2 diabetes in a general Japanese population: the Hisayama study. Diabetes Care. 2009;32:2288–93.
American Diabetes Association. 6.Glycemic targets. Diabetes Care. 2017;40:S48–56.
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86.
Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–17.
Nakayama M, Sato T, Miyazaki M, Matsushima M, Sato H, Taguma Y, et al. Increased risk of cardiovascular events and mortality among non-diabetic chronic kidney disease patients with hypertensive nephropathy: the Gonryo study. Hypertens Res. 2011;34:1106–10.
Djamali A, Kendziorski C, Brazy PC, Becker BN. Disease progression and outcomes in chronic kidney disease and renal transplantation. Kidney Int. 2003;64:1800–7.
Iseki K, Ikemiya Y, Iseki C, Takishita S. Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 2003;63:1468–74.
Wen CP, Matsushita K, Coresh J, Iseki K, Islam M, Katz R, et al. Relative risks of chronic kidney disease for mortality and end-stage renal disease across races are similar. Kidney Int. 2014;86:819–27.
Tonelli M, Muntner P, Lloyd A, Manns BJ, James MT, Klarenbach S, et al. Using proteinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: a cohort study. Ann Intern Med. 2011;154:12–21.
Black C, Sharma P, Scotland G, McCullough K, McGurn D, Robertson L, et al. Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis. Health Technol Assess. 2010;14:1–184.
Furuichi K, Yuzawa Y, Shimizu M, Hara A, Toyama T, Kitamura H, et al. Nationwide multicentre kidney biopsy study of Japanese patients with type 2 diabetes. Nephrol Dial Transplant. 2017;. doi:10.1093/ndt/gfw417 (Epub ahead of print).
Shima K, Chujo K, Yamada M, Komatsu M, Noma Y, Mizuguchi T. Lower value of glycated haemoglobin relative to glycaemic control in diabetic patients with end-stage renal disease not on haemodialysis. Ann Clin Biochem. 2012;49:68–74.
Vos FE, Schollum JB, Coulter CV, Manning PJ, Duffull SB, Walker RJ. Assessment of markers of glycaemic control in diabetic patients with chronic kidney disease using continuous glucose monitoring. Nephrology (Carlton). 2012;17:182–8.
Acknowledgements
Contributors: Gonryo Study investigators: Yoshio Taguma and Toshinobu Sato (Japan Community Health care Organization Sendai Hospital), Masato Matsushima (Tokyo Jikeii Medical University), Katsuya Obara (Tohoku Kosai Hospital), Isao Kurihara (Tohoku Kosai Miyagino Hospital), Yasumichi Kinoshita, Kazuto Sato, and Tasuku Nagasawa (Japanese Red Cross Ishinomaki Hospital), Kazuro Kaise and Yoshihide Arihara (Sendai Medical Center), Akira Sugiura and Masahiro Miyata (Osaki Citizen Hospital), Kazuhisa Takeuchi (Koujinkai Central Clinic), Kenji Nakayama and Naoki Akiu (Sendai City Hospital), and Tetsuya Otaka (Katta General Hospital). The authors express special thanks to Ms. Yumi Yamaguchi and Ms. Ayuko Momoi for administrative assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the institutional review board of Tohoku University, Graduate School of Medicine (No. 2006-10), and the study protocol was approved by the respective participating hospitals. All patients provided written informed consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Support and funding
Astellas Pharmaceutical. Miyagi Kidney Foundation.
Other disclosures
None.
About this article
Cite this article
Iwai, T., Miyazaki, M., Yamada, G. et al. Diabetes mellitus as a cause or comorbidity of chronic kidney disease and its outcomes: the Gonryo study. Clin Exp Nephrol 22, 328–336 (2018). https://doi.org/10.1007/s10157-017-1451-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-017-1451-4