Abstract
Background
Preoperative chemoradiotherapy (CRT) is a standard treatment for stage II/III esophageal cancer. Preoperative chemotherapy is also considered a standard treatment for stage II/III esophageal squamous cell carcinoma (ESCC) in patients who undergo radical lymph node dissection. We conducted a feasibility study of preoperative CRT with cisplatin plus 5-fluorouracil (CF) and elective lymph node irradiation followed by esophagectomy with radical lymph node dissection in patients with stage II/III ESCC.
Methods
Patients with clinical stage II/III, excluding T4, ESCC (International Union Against Cancer TNM classification system, 6th edition) were eligible. Chemotherapy comprised two courses of CF infusion repeated after 4-weeks. Radiation therapy was concurrently administered to the primary tumor, metastatic lymph nodes, and regional lymph nodes at a dose of 41.4 Gy. After the completion of CRT, transthoracic esophagectomy with 2–3 fields lymphadenectomy was performed. The primary endpoint was the completion rate of protocol treatment with R0 resection.
Results
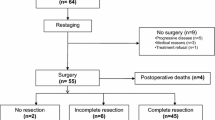
Thirty-one eligible patients were enrolled. During CRT, the most common grade 3 or 4 toxicities were leukopenia (65%), neutropenia (65%), anemia (13%), thrombocytopenia (13%), febrile neutropenia (13%), anorexia (16%), esophagitis (16%) and hyponatremia (16%). Thirty patients (96.8%) underwent surgery. One patient received palliative chemotherapy because of appearance of lung metastasis during CRT. The completion rate of protocol treatment was 93.5% (29/31). There was one treatment-related death after surgery. Pathological complete response was achieved in 42% (13/30).
Conclusion
Preoperative CRT with CF and elective lymph node irradiation showed an acceptable toxicity and promising activity especially in ESCC.


Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386
Tachimori Y, Ozawa S, Numasaki H et al (2016) Comprehensive registry of esophageal cancer in Japan, 2009. Esophagus 13:110–137
Sjoquist KM, Burmeister BH, Smithers BM et al (2011) Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 12:681–692
Kidane B, Coughlin S, Vogt K et al (2015) Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database Syst Rev 19:CD001556
Chan KKW, Saluja R, Delos Santos K et al (2018) Neoadjuvant treatments for locally advanced, resectable esophageal cancer: a network meta-analysis. Int J Cancer 143:430–437
van Hagen P, Hulshof MC, van Lanschot JJ et al (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366:2074–2084
Mariette C, Dahan L, Mornex F et al (2014) Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 32:2416–2422
Stahl M, Stuschke M, Lehmann N et al (2005) Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 23:2310–2317
Urba SG, Orringer MB, Turrisi A et al (2001) Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 19:305–313
Akiyama H, Tsurumaru M, Udagawa H et al (1994) Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 220:364–372
Inokuchi K (1992) Milestones along the road to improvement of results in the treatment of squamous cell carcinoma of the esophagus. In: Color atlas of surgical anatomy for esophageal cancer. Springer, Tokyo, pp 1–8
Ando N, Kato H, Igaki H et al (2012) A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 19:68–74
Kato K, Nakajima TE, Ito Y et al (2013) Phase II study of concurrent chemoradiotherapy at the dose of 50.4 Gy with elective nodal irradiation for Stage II-III esophageal carcinoma. Jpn J Clin Oncol 43:608–615
Japanese Society for Esophageal Diseases (2004) Guidelines for clinical and pathologic studies on carcinoma of the esophagus ninth edition: part II. Esophagus 1:107–125
Tepper J, Krasna MJ, Niedzwiecki D et al (2008) Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 26:1086–1092
Klevebro F, Alexandersson von GDG, Wang N et al (2016) A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol 27:660–667
Nakamura K, Kato K, Igaki H et al (2013) Japan esophageal oncology group/Japan clinical oncology group. Jpn J Clin Oncol 43:752–755
van Meerten E, Muller K, Tilanus HW et al (2006) Neoadjuvant concurrent chemoradiation with weekly paclitaxel and carboplatin for patients with oesophageal cancer: a phase II study. Br J Cancer 94:1389–1394
Bosset JF, Gignoux M, Triboulet JP et al (1997) Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 337:161–167
Akutsu Y, Kato K, Igaki H et al (2016) The Prevalence of overall and initial lymph node metastases in clinical t1n0 thoracic esophageal cancer: from the results of JCOG0502, a prospective multicenter study. Ann Surg 264:1009–1015
Ferri LE, Ades S, Alcindor T et al (2012) Perioperative docetaxel, cisplatin, and 5-fluorouracil (DCF) for locally advanced esophageal and gastric adenocarcinoma: a multicenter phase II trial. Ann Oncol 23:1512–1517
Hara H, Tahara M, Daiko H et al (2013) Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci 11:1455–1460
Stahl M, Walz MK, Stuschke M et al (2009) Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 27:851–856
Nakamura K, Kato K, Igaki H et al (2013) Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol 43:752–755
Acknowledgements
We express our appreciation to Mr. Nagai, Ms. Tada, Ms. Usami, Ms. Sakamoto, Ms. Ayabe, and Ms. Shinogi for data collection and management.
Funding
This study was also supported by National Cancer Center Research and Development Funds (23-A-16, 23-A-18 and 26-A-4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No author has any conflict of interest.
About this article
Cite this article
Hashimoto, J., Kato, K., Ito, Y. et al. Phase II feasibility study of preoperative concurrent chemoradiotherapy with cisplatin plus 5-fluorouracil and elective lymph node irradiation for clinical stage II/III esophageal squamous cell carcinoma. Int J Clin Oncol 24, 60–67 (2019). https://doi.org/10.1007/s10147-018-1336-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-018-1336-x