Abstract
Background
The prognostic significance of CC chemokine receptor type 7 (CCR7) for survival of patients with gastric cancer remains controversial. To investigate the impacts of CCR7 on clinicopathological findings and survival outcome in gastric cancer, we performed a meta-analysis.
Methods
A comprehensive search in PubMed, Embase, the Cochrane Library, and the CNKI database (1966 to November 2015) was undertaken for relevant studies. The relative risk and hazard ratios with their 95 % confidence intervals were used as measures to investigate the correlation between CCR7 expression and clinicopathological findings and overall survival rate. Sensitivity analysis was conducted to assess the stability of outcomes.
Results
Fifteen eligible studies comprising 1697 participants were included in our analysis. The pooled relative risks indicated CCR7 expression was significantly associated with deeper tumor invasion [0.61, 95 % confidence interval (CI) 0.45–0.84, p = 0.003], advanced stage (0.47, 95 % CI 0.32–0.69, p < 0.001), vascular invasion (2.12, 95 % CI 1.20–3.73, p = 0.009), lymph node metastasis (2.00, 95 % CI 1.48–2.70, p < 0.001), and lymphatic invasion (1.98, 95 % CI 1.43–2.72, p < 0.001) but not with age, tumor size, and histological type. The pooling of hazard ratios showed a significant relationship between positive CCR7 expression and worse 5-year overall survival rate (0.46, 95 % CI 0.31–0.70, p < 0.001).
Conclusions
Our meta-analysis indicated high CCR7 expression is likely to be a negative clinicopathological prognostic factor for patients with gastric cancer and to predict a worse long-term survival outcome.
Similar content being viewed by others
Introduction
As a G-protein-coupled and seven-span transmembrane protein, CC chemokine receptor type 7 (CCR7) is expressed in T cells and mature dendritic cells [1, 2]. Secondary lymphoid chemokine (CCL21) and EB11-ligand chemokine (CCL19) are its two high-affinity ligands. CCR7 binding with its ligands could promote immune cell viability and induce homing of immune cells to secondary lymphoid organs in immunological and inflammatory processes [3, 4]. Studies revealed that several chemokines and their receptors, such as stromal cell derived factor 1/CXCR4 and CCL1/CCR8, can promote cancer cell dissemination and metastasis [5]. CCR7, the chemokine receptor also expressed in multiple carcinomas, facilitates lymph node metastasis [6]. Many studies have been conducted to understand the relationship between CCR7 expression and clinicopathological features and 5-year overall survival (OS) rate, but a unanimous conclusion has yet to be reached. The prognosis of CCR7 expression in gastric cancer reported by different researchers was contradictory [7–10].
To address this issue, we pooled results of relevant articles through meta-analysis to investigate the value of CCR7 as a prognostic marker for OS rate and determine the relationship between CCR7 expression and clinicopathological features of gastric cancer.
Methods
Data source
A systematic literature search of PubMed, the Cochrane Library, Embase, and the CNKI database (http://www.cnki.net) from 1966 to November 2015 was undertaken with restriction to English and Chinese. The strategy used was to search for the following words in relevant articles: “gastric” or ”stomach”; “neoplasm,” “cancer,” or “malignant tumor”; and “CCR7” or “chemokine receptor 7.” We used the function “related article” in the database, and this was applied to search relevant articles. Articles in the reference lists were searched if they were likely to be of relevance to this topic. Two investigators (P. Du and Y. Liu) performed the literature search independently.
Inclusion criteria
Patients in the study or the study properties had to fulfill the following criteria to be were included in the analysis.(1) either randomized controlled studies or observational studies were allowed; (2) patients with pathologically confirmed gastric cancer who underwent detection of CCR7 in tumor cells; (3) studies evaluating the relationship between CCR7 expression and parameters such as clinicopathological features and/or 5-year OS rate; and (4) studies containing sufficient published data to determine an estimate of the relative risk (RR), the hazard ratio (HR), and their 95 % confidence intervals (CIs) calculated by multivariate analyses with a Cox proportional hazards regression model. Studies were excluded for if they (1) had no dichotomous groups with CCR7 expression and (2) investigated CCR7 in peripheral lymphocytes of gastric carcinoma and its clinical significance, rather than in tumor cells. When there were multiple publications regarding the same patient group, only one publication was included.
Data extraction
Two investigators (P. Du and Y. Liu) reviewed the abstracts and full-text articles that were obtained. They sought help from a third party (G. Huang) when they could not reach a consensus. Disagreements were resolved by consultation. Studies were included in the meta-analysis only if all three reviewers agreed on their inclusion.
Quality assessment
In accordance with the Newcastle–Ottawa scale [11], quality assessment mainly focused on selection (representativeness, selection of the nonexposed, ascertainment of exposure, and outcome of interest), comparability of cohorts on the basis of the design or analysis, and the outcome (assessment and follow-up) of the original studies. Assessment was performed independently by two reviewers (P. Du and Y. Liu). Discrepancies were resolved by consensus in the presence of a third investigator (G. Huang).
Statistical analysis
The RR with its 95 % CI was chosen to investigate the association between clinicopathological features and CCR7 expression in gastric tumor cells. The HR, which takes into account the number of events and the timing of events, with its 95 % CI was adopted to pool the studies involved in comparison with the 5-year survival rates. Statistically significant heterogeneity was defined as a χ 2 p value of less than 0.1 or an I 2 statistic of more than 50 %. The inverse variance method and the Mantel–Haenszel method were randomly adopted and a fixed effects model was used.
STATA version 12.0 (Stata, College Station, TX, USA) was used to conduct the analyses. We used the “metainf” command of STATA to assess the stability of the original data. The possibility of publication bias was assessed by the Begg adjusted rank correlation test with visual inspection of the funnel plot. The trim and fill method was used in this meta-analysis to further assess the possible effect of publication bias [12]. This method considers the possibility of hypothetical “missing” studies that might exist, imputes their RRs, and recalculates a pooled RR that incorporates the hypothetical missing studies as though they actually existed.
Results
Study selection
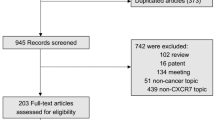
From PubMed, the Cochrane Library, Embase, and the CNKI database, we obtained 1548 relevant articles. After excluding irrelevant articles by reviewing the titles and abstracts of those studies, two investigators (P. Du and Y. Liu) reviewed the full text version of the remaining 30 articles independently in accordance with the inclusion and exclusion criteria. Finally, 15 articles [7–10, 13–23] comprising data on 1697 participants met the requirement of the analysis. The detailed search steps are described in Fig. 1.
Study characteristics
All of studies we included were published between 2002 and 2015, and the number of patients in each study ranged from 30 to 307. The proportion of patients with high CCR7 expression ranged from 20 to 72 %. To detect the expression of CCR7, all studies used immunohistochemistry, except for one study, which used reverse transcription polymerase chain reaction, but its percentage of CCR7 expression was consistent with that of the other studies. Clinicopathological features included the age, tumor size, T (tumor) category, stage, vascular invasion, lymphatic invasion, lymph node metastasis, and histological findings. There was a difference in the pooling of HRs. Seven studies reported survival of patients. Four of them reported the value of the HRs and 95 % CIs calculated by multivariate analyses with a Cox proportional hazards regression model. Three of them only reported results that were calculated by Kaplan–Meier estimates (log-rank test), which falls under the univariate analysis category. After consulting the third party, P. Du and Y. Liu decided to exclude the studies reported by Deguchi et al. [15], Mashino et al. [22], and Kwak et al. [7] because it was inappropriate to pool HRs calculated by two substantially different statistical methods. These two investigators extracted basic data, and these are shown in Table 1.
Assessment of quality
In accordance with to the Newcastle–Ottawa scale, the patients in the studies were selected rationally. The final diagnoses were validated by postoperative pathology examination. All patients in different groups came from the same demographic. The criteria dividing patients into different groups were fixed. Most of the articles reported that patients in both groups were comparable. Patients’ clinical data were collected from the permanent institutions of the respective hospitals. In each study, the methods of ascertainment for cases and the immunohistochemistry test used to clarify the expression of CCR7 in tumor cells were fixed. What cannot be assessed in each study is that rare studies described the respondents of two groups.
Correlation of CCR7 expression with clinicopathological parameters
CCR7 expression was not associated with age (pooled RR 1.12, 95 % CI 0.90–1.39, p = 0.309, random effect), tumor size (pooled RR 1.00, 95 % CI 0.67–1.49, p = 0.989, random effect), or histological type (pooled RR 1.21, 95 % CI 0.91–1.61, p = 0.189, random effect). However, gastric cancers with CCR7 expression were associated with several clinical parameters, such as depth of tumor invasion(pooled RR 0.61, 95 % CI 0.45–0.84, p = 0.003, random effect), stage of tumor (pooled RR 0.47, 95 % CI 0.32–0.69, p < 0.001, random effect), vascular invasion (pooled RR 2.12, 95 % CI 1.20–3.73, p = 0.009, random effect), lymph node metastasis (pooled RR 2.00, 95 % CI 1.48–2.70, p < 0.001, random effect), and lymphatic invasion (pooled RR 1.98, 95 % CI 1.43–2.72, p < 0.001, random effect) (Figs. 2, 3).
Impact of CCR7 expression on 5-year OS
The relationship between CCR7 expression and gastric cancer prognosis is illustrated in Fig. 4. High expression of CCR7 was significantly associated with a worse 5-year OS rate (pooled HR 0.46, 95 % CI 0.31–0.70, p < 0.001, fixed effect).
Publication bias
The p values from Begg’s tests indicated that there was no significant publication bias for pooled age (p = 0.452), tumor size (p = 0.812), vascular invasion (p = 0.680), lymphatic invasion (p = 0.322), and histological type (p = 0.214) (Fig. 5). But the publication bias for pooled T category (p = 0.003), stage (p < 0.001), and lymph node metastasis was significant (p = 0.001). To assess the influence of bias in the three above-mentioned analyses, we undertook a sensitivity analyses using the trim and fill method [24], which conservatively imputes hypothetical negative unpublished studies to mirror the positive results that cause funnel plot asymmetry. All of the subsequent analyses revealed that the filled outcomes of T category (p = 0.002), stage (p < 0.001), and lymph node metastasis (p = 0.006) were unchanged (Fig. 6).
Discussion
CCR7 expression has been detected in a variety of human cancer cells and has been correlated with increased metastasis, such as breast cancer [25] and colorectal cancer [26] metastasis. However, the prognostic and predictive value of CCR7 in gastric cancer is still controversial. To our knowledge, this present meta-analysis is the first study to systematically evaluate the association between chemokine receptor CCR7 and clinicopathological features and the prognostic factors in gastric cancer. In the present study, a pooled analysis of 15 clinical studies which detected CCR7 expression in tumor cells revealed a negative prognostic outcome in patients expressing high levels of CCR7, and showed a significant correlation between CCR7 expression and deeper tumor invasion, advanced stage, vascular invasion, lymphatic invasion, and lymph node metastasis.
The mechanism of CCR7–CCL19/CCL21 leading to dissemination and metastasis formation is mainly as follows. CCR7 is naturally a homeostatic chemokine receptor and is expressed on various subtypes of immune cells that migrate to and within lymphoid organs. The CCR7–CCL19/CCL21 axis is well characterized for its crucial role in the formation of secondary lymphoid structures under physiological conditions mainly through orchestration of the recruitment of immune cells to these structures [27]. The chemokine CCL21 is immobilized on lymphatic endothelial cells and forms a gradient that gradually decreases in the direction of the interstitium [28]. Cancer cells with upregulated CCR7 disseminate from the primary tumor presumably by sensing the immobilized CCL21 gradient and actively migrate toward the next lymphatic vessel and the T-cell zone of lymph nodes. In addition to contributing to tumor cell survival by activating the phosphatidylinositol-3-kinase/Akt signaling pathway [29], the CCL21/CCR7 axis can upregulate matrix metalloproteinase 9 [30], E-cadherin, Slug, vimentin, and N-cadherin [31], which are considered as markers of epithelial–mesenchymal transition (EMT); that is to say, CCR7 can stimulate cancer cells to acquire an EMT phenotype. Accumulating evidence has shown that the EMT is a pathological process contributing to cancer progression, particularly cancer invasion, dissemination, and metastasis [32]. Coincidentally, CCL19 and CCL21 are abundant in a carcinoma microenvironment, because CCL19 and CCL21 are produced by not only lymphatic endothelium but also cancer cells and stromal cells in carcinoma tissue and pericancerous tissue [33–35], which leads to EMT of tumor cells and further promotes the metastasis of tumor cells.
On the basis of the results of the above-mentioned in vitro experiment and the significant relationship between high CCR7 expression and worse clinicopathological features, we have been able to deduce that CCR7 may be associated with a negative OS rate. As expected, our pooling of HRs confirmed our prediction that high CCR7 expression is a significantly negative prognostic factor. In four included studies, the involvement of other prognostic factors, such as TNM stage, lymph node metastasis, and differentiation, was eliminated through statistical methods of adjustment in multivariate analyses. Subsequently, all of the patients underwent curative gastrectomy with necessary lymphadenectomy. Good consistency increased the reliability of our outcomes. However, there were some flaws in our pooling the HRs. All of the articles that were searched did not offer information regarding postoperative chemotherapy/radiotherapy, and the number of censored patients in the study reported by Ishigami et al. [13] was unclear; hence these factors may have caused some inaccuracies in the outcomes. Kwak et al. [7] concluded that the prognosis of patients with a CCR7-positive tumor was better than that of patients with a CCR7-negative tumor. However, these are results of only univariate analyses, and this shows that more relevant trials are still needed to testify the significance of CCR7 with regard to the long-term survival rate.
Other limitations of this meta-analysis must also be considered. Firstly, all the studies came from Asia. This geographical limitation can result in the conclusion that the meta-analysis is not reflective of the function of CCR7, especially in the West. Secondly, the heterogeneity among the studies cannot be ignored. This may be caused by the difference in immunohistochemistry materials and methods, along with the different methods of expression evaluation used. Thirdly, Begg’s test showed publication bias in three analyses is significant. The trim and fill sensitivity analysis did not change the general result, suggesting that the association is not an artifact of unpublished studies with negative findings; nevertheless, that possibility is not fully excluded by this method. Fourthly, the sample size in many of the studies was small. Small studies can reflect clinical heterogeneity if small trials were more careful in selecting patients [36], but can also distort the results of meta-analyses. More large studies are still needed to explore the prognostic function of CCR7 for people in different geographical areas and of different races to clarify the prognostic value of CCR7 in predicting the long-term survival rate and the disease-free survival rate in patients with gastric cancer, to formulate reliable and unique methods of detecting the expression of CCR7, and to explore the curative effect of therapy targeting CCR7 and assess its side effects.
In conclusion, our meta-analysis indicated the expression of CCR7 is a negative prognostic factor in gastric cancer. CCR7 expression has a strong correlation with deeper tumor invasion and more frequent advanced stage disease, lymph node metastases, and vascular and lymphatic invasion, and subsequently predicts a worse long-term survival outcome.
References
Hirao M, Onai N, Hiroishi K, Watkins SC, Matsushima K, Robbins PD, et al. CC chemokine receptor-7 on dendritic cells is induced after interaction with apoptotic tumor cells: critical role in migration from the tumor site to draining lymph nodes. Cancer Res. 2000;60:2209–17.
Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait-Yahia S, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–86.
Baekkevold ES, Yamanaka T, Palframan RT, Carlsen HS, Reinholt FP, von Andrian UH, et al. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J Exp Med. 2001;193:1105–12.
Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci U S A. 1998;95:258–63.
Das S, Sarrou E, Podgrabinska S, Cassella M, Mungamuri SK, Feirt N, et al. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J Exp Med. 2013;210:1509–28.
Zlotnik A. Chemokines and cancer. Int J Cancer. 2006;119:2026–9.
Kwak MK, Hur K, Park DJ, Lee HJ, Lee HS, Kim WH, et al. Expression of chemokine receptors in human gastric cancer. Tumour Biol. 2005;26:65–70.
Arigami T, Natsugoe S, Uenosono Y, Yanagita S, Arima H, Hirata M, et al. CCR7 and CXCR4 expression predicts lymph node status including micrometastasis in gastric cancer. Int J Oncol. 2009;35:19–24.
Zhou S, Shen Z, Wang Y, Ma H, Xu S, Qin J, et al. CCR7 expression and intratumoral FOXP3+ regulatory T cells are correlated with overall survival and lymph node metastasis in gastric cancer. PLoS One. 2013;8:e74430.
Zhang J, Zhou Y, Yang Y. CCR7 pathway induces epithelial-mesenchymal transition through up-regulation of Snail signaling in gastric cancer. Med Oncol. 2015;32:467.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Ishigami S, Natsugoe S, Nakajo A, Tokuda K, Uenosono Y, Arigami T, et al. Prognostic value of CCR7 expression in gastric cancer. Hepatogastroenterology. 2007;54:1025–8.
Yan C, Zhu ZG, Yu YY, Ji J, Zhang Y, Ji YB, et al. Expression of vascular endothelial growth factor C and chemokine receptor CCR7 in gastric carcinoma and their values in predicting lymph node metastasis. World J Gastroenterol. 2004;10:783–90.
Deguchi K, Ichikawa D, Soga K, Watanabe K, Kosuga T, Takeshita H, et al. Clinical significance of vascular endothelial growth factors C and D and chemokine receptor CCR7 in gastric cancer. Anticancer Res. 2010;30:2361–6.
Wang WN, Chen Y, Zhang YD, Hu TH. The regulatory mechanism of CCR7 gene expression and its involvement in the metastasis and progression of gastric cancer. Tumour Biol. 2013;34:1865–71.
Zhao J, Wang G, Sun M. Expression and clinical significance of chemokine receptor CCRT in gastric carcinoma and its corresponding lymph node metastasis. Chin J Lab Diagn. 2007;11:468–70.
Chen Z. The expression and significance of chemokin receptor CCR6, CCR7 in gastric cancer [master's thesis]. Dalian Medical University; 2009.
Wang T. Expressions of chemokine receptors CXCR1, CXCR2 and CCR7 in gastic carcinoma and their relevance with metastasis [master's thesis]. Dalian Medical University; 2005.
Wen G, Guo Y. The expression of chemokine receptor CCR7 and CXCR4 in gastric cancer. Guangdong Med J. 2006;27:968–70.
Yan Y, Wu R, Shen X, Yang P, Wang Y. Overexpression of chemokine receptor CCR7 in gastric cancer tissue and cell lines. Chin J Gastroenterol. 2010;15:423–5.
Mashino K, Sadanaga N, Yamaguchi H, Tanaka F, Ohta M, Shibuta K, et al. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002;62:2937–41.
Ma H, Gao L, Li S, Qin J, Chen L, Liu X, et al. CCR7 enhances TGF-β1-induced epithelial-mesenchymal transition and is associated with lymph node metastasis and poor overall survival in gastric cancer. Oncotarget. 2015;6:24348–60.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63.
Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6.
Gunther K, Leier J, Henning G, Dimmler A, Weissbach R, Hohenberger W, et al. Prediction of lymph node metastasis in colorectal carcinoma by expression of chemokine receptor CCR7. Int J Cancer. 2005;116:726–33.
Comerford I, Harata-Lee Y, Bunting MD, Gregor C, Kara EE, McColl SR. A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev. 2013;24:269–83.
Weber M, Hauschild R, Schwarz J, Moussion C, de Vries I, Legler DF, et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339:328–32.
Wang J, Zhang X, Thomas SM, Grandis JR, Wells A, Chen ZG, et al. Chemokine receptor 7 activates phosphoinositide-3 kinase-mediated invasive and prosurvival pathways in head and neck cancer cells independent of EGFR. Oncogene. 2005;24:5897–904.
Guo N, Liu F, Yang L, Huang J, Ding X, Sun C. Chemokine receptor 7 enhances cell chemotaxis and migration of metastatic squamous cell carcinoma of head and neck through activation of matrix metalloproteinase-9. Oncol Rep. 2014;32:794–800.
Li F, Zou Z, Suo N, Zhang Z, Wan F, Zhong G, et al. CCL21/CCR7 axis activating chemotaxis accompanied with epithelial-mesenchymal transition in human breast carcinoma. Med Oncol. 2014;31:180.
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90.
Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11:526–38.
Sperveslage J, Frank S, Heneweer C, Egberts J, Schniewind B, Buchholz M, et al. Lack of CCR7 expression is rate limiting for lymphatic spread of pancreatic ductal adenocarcinoma. Int J Cancer. 2012;131:E371–81.
Mumtaz M, Wagsater D, Lofgren S, Hugander A, Zar N, Dimberg J. Decreased expression of the chemokine CCL21 in human colorectal adenocarcinomas. Oncol Rep. 2009;21:153–8.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Acknowledgments
We greatly appreciate the help of Adam J Sullivan, Department of Biostatistics, Brown University, USA, and Zhang Dongfeng, Department of Epidemiology and Health Statistics, Medical College of Qingdao University, China, who provided crucial guidance in our revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No grants, equipment, or drugs were provided to support this work. No funding from any organization was received for this work. The authors declare that they have no conflict of interest.
Ethical standards
This article does not contain any studies with human or animal subjects performed by any of the authors.
Rights and permissions
About this article
Cite this article
Du, P., Liu, Y., Ren, H. et al. Expression of chemokine receptor CCR7 is a negative prognostic factor for patients with gastric cancer: a meta-analysis. Gastric Cancer 20, 235–245 (2017). https://doi.org/10.1007/s10120-016-0602-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-016-0602-8