Abstract
Background
A partially covered self-expandable metallic stent (PCSEMS) is of proven benefit in palliation of unresectable or inoperable malignant gastric outlet obstruction. However, its use in patients with benign anastomotic stricture after laparoscopy-assisted gastrectomy (LAG) is not well established.
Methods
Patients who between May 2007 and June 2012 underwent PCSEMS placement for management of benign gastrointestinal obstruction after LAG were included in this retrospective analysis. The primary outcomes were the technical success and clinical success of the PCSEMS. The secondary outcomes were procedure-related complications and PCSEMS dysfunction.
Results
Eleven patients (six women, five men, mean age 53.5 years, range 15–76 years) underwent successful placement of a PCSEMS for management of benign anastomotic strictures after LAG and were followed-up for a mean of 20.6 months (range 7.9–55.6 months). The mean gastric outlet obstruction scoring system (GOOSS) score was 0.36 before PCSEMS placement and 1.55 (p = 0.010) 24–48 h after PCSEMS placement. All of the patients were able to tolerate a solid diet (GOOSS score 3) after 1 week. There were no major or minor procedure-related complications. Stent dysfunction occurred in four patients (three distal migrations, one proximal migration), and stent removal was successful in all of the remaining patients after a mean of 2.0 months (1.1–3.0 months). Obstructive symptoms recurred in two patients (one after proximal migration, one after stent removal) and were treated successfully with PCSEMS reinsertion and balloon dilation.
Conclusions
A PCSEMS may be a feasible and effective option for management of benign anastomotic strictures after LAG which could avoid secondary surgery.
Similar content being viewed by others
Introduction
Since its introduction in 1991 in Japan, minimally invasive surgery for gastric cancer has been an important treatment modality. The practice of surgical techniques in laparoscopy-assisted gastrectomy (LAG) as a better alternative to open gastrectomy has been increasing [1, 2], and reduced blood loss, shorter time to resumption of oral intake, and shortened hospital stay have been reported [3]. However, because of the difficult reconstruction technique, surgical morbidity and mortality after LAG are considerable, and several studies have reported incidences of surgical morbidity ranging from 9.8 to 26.7 % and surgical mortality ranging from 0 to 3.3 % [3–6]. In addition, in particular, the incidence of anastomotic stricture after LAG was reported to be 1.3–2.9 % [7–9], and the presence of an anastomotic complication is known to contribute to the long-term outcome of patients [8].
The traditional management of anastomotic stricture was revisional surgery, which has been associated with high morbidity in already compromised and nutritionally depleted patients [10]. Recently, use of endoscopic balloon dilation of strictures has resulted in significantly improved stricture outcomes by largely avoiding surgical revision. However, it still carries a risk of perforation and stricture recurrence [11, 12]. Adequate postoperative management of the various complications, including anastomotic strictures, is important, and, in particular, appropriate performance of endoscopic intervention could result in reduced hospital stay and prevention of more serious complications requiring reoperation.
A partially covered self-expandable metallic stent (PCSEMS) is of proven benefit in patients with inoperable malignant gastric outlet obstruction or in those who are unsuitable for surgery owing to the presence of significant comorbid obstruction [13–15], and the use of a PCSEMS for management of benign gastrointestinal (GI) disorders, including esophageal variceal bleeding, benign upper GI tract leaks, or perforation, is increasing [16–19]; its use has also been attempted for management of several benign gastrointestinal strictures, including Crohn’s disease strictures, benign esophageal obstruction, anastomotic complications after bariatric surgery, or anastomotic complications after colorectal resection [20–24]. However, its use for palliation in patients with benign anastomotic stricture after LAG is not well established. Therefore, this study assessed the feasibility, efficacy, and safety of the PCSEMS in the management of benign anastomotic strictures after LAG.
Materials and methods
Patients
Patients who underwent PCSEMS placement from May 2007 to June 2012 for management of benign proximal GI tract obstruction after LAG were identified by review of electronic medical records and the endoscopy procedure registry at Seoul National University Bundang Hospital. Data regarding patient demographics, indication for LAG, type of gastric resection, type of anastomosis, procedure outcome, and procedure-related complications were collected retrospectively. During the study period, 1,328 laparoscopic gastrectomies were performed in our institution. Among those, approximately 90 % were distal gastrectomy and 10 % were total gastrectomy. Billroth I anastomosis procedures were performed in approximately 70 % of patients who had received distal gastrectomy. The study protocol was approved by the Institutional Review Board of Seoul National University Bundang Hospital (Institutional Review Board no. B-1211/180-111) and conformed to the ethical guidelines of the Declaration of Helsinki, 1964, as revised in 2004. The requirement for informed consent was waived.
Procedures
Before stent insertion, the patients underwent conventional forward-viewing upper endoscopy for assessment of the degree, nature, and site of the obstruction. Before stent placement was attempted, a nasogastric aspiration tube was placed in all patients for 24 h in order to improve the endoscopic view and to reduce the risk of vomiting and aspiration during the procedure. PCSEMS placement was performed by a single endoscopist using a two-channel endoscope (GIF-2T200, Olympus, Japan) under fluoroscopic guidance. The stricture was traversed using a 300-cm-long, 0.035-in. Superstiff guidewire (Microvasive Endoscopy, Boston Scientific, Natick, MA, USA), and the PCSEMS was loaded over the guidewire and passed through the accessory channel of the endoscope. All of the PCSEMS (BONASTENT® pyloric/duodenal covered stents, Standard Sci-Tech, Seoul, Korea) were covered with a membrane and had uncovered flares on both ends. The stent diameter was 20 mm and the length ranged from 60 to 100 mm, depending on the case. Estimation of the stricture length was based on the fluoroscopic image, and an attempt was made to select stents that would give 1 or 2 cm of free margin above and below strictures after insertion, by taking into account the shortening that occurs during stent expansion. After stent placement, patients were allowed to drink 24 h after the procedure, and if liquids were well tolerated, to resume a soft, low-residue diet. Follow-up endoscopy was performed in all patients within 1–2 months after placement of the PCSEMS, and stent removal was attempted according to the decision of the treating physician.
Study end points
The primary end points were the technical success and clinical success of the PCSEMS. The secondary end points were procedure-related complications and PCSEMS dysfunctions. Technical success was defined as satisfactory deployment of the PCSEMS in the correct position across the obstructing lesion and confirmation of patency using a combination of endoscopy and fluoroscopy. Clinical success was defined as an improvement in the ability to tolerate an oral dietary intake after stenting as assessed using the gastric outlet obstruction scoring system (GOOSS) score (Table 1) [25]. Major complications were defined as complications requiring a repeated endoscopic intervention or hospitalization. Minor complications were defined as mild to moderately severe complications, which could be treated conservatively with no need for hospitalization. PCSEMS dysfunction was defined as the obstruction or migration of the PCSEMS requiring additional intervention.
Statistical analysis
The data are reported as a number (and a percentage) for categorical variables or as the mean (and the range) for continuous variables. Analysis of the pre-PCSEMS placement and post-PCSEMS placement GOOSS scores was performed using the Wilcoxon signed-rank test. A p value of less than 0.05 was considered statistically significant. Analysis was performed using Statistical Package for the Social Sciences, version 18.0 for Windows (SPSS, Chicago, IL, USA).
Results
Clinical characteristics of patients
Eleven patients with benign upper GI tract obstruction after LAG underwent PCSEMS placement. A summary of the cases is shown in Table 2. Of the 11 patients, five were male and six were female, with a mean age of 53.5 years (range 15–76 years). The indications for LAG were early gastric cancer in nine patients (81.8 %), advanced gastric cancer in one patient, and gastric duplication cyst rupture in one patient. Obstruction of the upper GI tract was caused by stricture of the anastomotic site in ten patients (90.9 %), who underwent total or subtotal gastrectomy. The other patient underwent laparoscopic gastric wedge resection and primary repair, and the cause of GI tract obstruction was torsion of the gastric outlet. In two cases, predilation of the stricture site using a 10-mm endoscopic balloon was required in order to allow passage of the guidewire/stent assembly. In one case, bougienage of the stricture site was performed a few days before PCSEMS placement.
Primary outcome assessment
Of the 11 patients, technical success was achieved in all patients who underwent PCSEMS placement (Fig. 1). The GOOSS scores before and after stent placement are shown in Fig. 2. Nine of 11 patients (81.8 %) showed improvement in their GOOSS score within 24–48 h, and the two remaining patients showed improvement in their GOOSS score after 1 week. In the comparison of the preprocedure and postprocedure GOOSS scores, a statistically significant improvement was observed in the overall GOOSS score (baseline vs 24–48 h after the procedure, p = 0.010, and baseline vs 1 week after the procedure, p = 0.002).
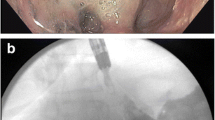
Images for a 15-year-old female patient with torsion of the gastric outlet after laparoscopy-assisted gastric wedge resection for a gastric duplication cyst. a Endoscopy performed 10 days after the operation shows gastric outlet obstruction caused by torsion. b–d A 10 cm × 2 cm partially covered self-expandable metallic stent was deployed 2 weeks after the operation. Vomiting was stopped within 72 h, and liquid diet was tolerable (gastric outlet obstruction scoring system score 0–1). The stent was removed 63 days after the procedure and the patient was followed-up for 2.9 years, and no symptom recurrence was observed
Secondary outcome assessment
No procedure-related major or minor complications were reported, and all of the patients were discharged within 1 week. PCSEMS dysfunction occurred in four patients, and the cause of stent dysfunction was PCSEMS migration in all four patients. The PCSEMS migrated distally in three patients and proximally in one patient (the stent came out orally during vomiting). All of the stent migrations occurred in patients who underwent laparoscopy-assisted distal gastrectomy with Billroth I anastomosis. However, despite stent migration, there was recurrence of upper GI tract obstruction in only one patient, and this was treated successfully with balloon dilation. A distally migrated PCSEMS was not observed during endoscopy or other imaging procedures, and it was presumed that it had passed spontaneously from the body.
Removal of the PCSEMS was performed successfully in all of the seven remaining patients within 34–89 days (median 63 days) after PCSEMS placement. Minor bleeding occurred in two patients, and it stopped spontaneously. The mean in-dwelling time of the PCSEMS was 55.8 days (range 29–89), except for three patients with distal migration. Recurrence of upper GI tract obstruction was observed in one patient at 22 days after removal of the PCSEMS, and was treated successfully with secondary PCSEMS placement. The patients were followed-up for a mean of 20.6 months (range 7.9–55.6 months), and no upper GI tract obstruction or cancer recurrence was observed.
Discussion
Several nonsurgical treatment modalities, including endoscopic balloon dilation and bougination, have been attempted for management of the anastomotic stricture after LAG [26, 27]. However, balloon dilation or bougination often requires a multisession procedure, and anastomotic strictures are commonly tenacious, tortuous, and angulated, and balloon dilation is often ineffective or only temporarily effective in such cases [26]. In this study, a PCSEMS was used for management of the anastomotic stricture after LAG, and it was effective and relatively safe in palliation of the obstructive symptoms. In addition, the PCSEMS could be easily removed after insertion without major complications.
In a previous study investigating the clinical efficacy of balloon dilation in benign anastomotic strictures after gastric surgery, two of seven patients (29 %) with anastomotic stricture after gastroduodenostomy did not respond to balloon dilation, and both patients eventually underwent revisional surgery [28]. On the other hand, in our study, all of the 11 patients, including eight patients who underwent distal gastrectomy with Billroth I anastomosis, responded to PCSEMS placement, and all of the patients were able to avoid revisional surgery.
Anastomotic strictures occur by various mechanisms, including inflammation and the subsequent development of fibrosis or edema at the anastomotic sites [29] or compression and twisting of the anastomosis. Balloon dilators deliver high radial force for only a short time and could be effective in anastomotic fibrosis. On the other hand, a PCSEMS delivers low radial force consistently, which could be effective not only in anastomotic fibrosis but also because it can withstand an external compressing force, twisting force, and/or elastic recoil. Because of the low radial force of the PCSEMS, there are theoretical benefits with regard to procedure-related perforation and bleeding.
A potential disadvantage of the PCSEMS is the possibility of stent migration and stricture recurrence, or, less commonly, bleeding, fistula, and perforation [26]. Stent migrations occurred in four of our 11 study patients (36.4 %); however, stent migration caused stricture recurrence in only one patient (9.1 %) and did not cause any other complications. Procedure-related bleeding, fistula, or perforation could also occur with balloon dilation [26], but these complications did not occur in our study patients, and the stent was removed successfully from all of the patients.
Stent dysfunction occurred in four patients, solely due to stent migration, and tissue ingrowth and overgrowth did not occur. All of the stent migrations occurred over 30 days after the stent placement. Despite stent migrations, the patency of the gastric outlet was maintained in most patients, and recurrence of the stricture after removal of the PCSEMS occurred in only one patient, who had undergone laparoscopy-assisted total gastrectomy and Roux-en-Y reconstruction. However, it was resolved after reinsertion of the PCSEMS and remained patent after more than 1 year of follow-up. A previous study reported that the postoperative complication rate of laparoscopy-assisted total gastrectomy is higher than that of laparoscopy-assisted distal gastrectomy (26.9 % vs 8.0 %, p < 0.001), particularly for anastomotic stricture (9.0 % vs 1.1 %, p < 0.001) [30]. Except for two patients, all other patients (nine patients, 81.8 %) remained recurrence free for follow-up periods of more than 10 months. The relatively small caliber of the stents could be one of the reasons for the stent migration. However, considering that the entire anastomotic stricture was a benign stricture, stent migration occurred over 30 days after the stent placement, and anastomotic stricture did not recur despite the stent migration in most of the patients, it is more likely that the stent migrations were due to the weakened friction force caused by the resolution of the stricture over time.
Until recently, anastomotic stricture was treated by endoscopic dilation, and there are plenty of reports on palliation of anastomotic stricture after esophagojejunostomy or gastrojejunostomy, whereas there are few reports on palliation of anastomotic stricture after gastroduodenostomy. However, in this study, most of the patients underwent distal gastrectomy with Billroth I anastomosis (gastroduodenostomy), and obstructive symptoms were resolved with PCSEMS placement.
The limitations of our study are that it was conducted in a single center and included a small number of patients. A single experienced endoscopist performed all of the PCSEMS procedures. Our excellent outcome may not be generalizable to other institutions or practitioners. All of the patients underwent LAG; however, the patients had diverse demographic and clinical features. The indications for gastrectomy were different between patients, and the anastomotic methods were also different. The data were collected retrospectively, and there were no comparison groups. Therefore, our result does not lead to a final conclusion. However, this is the first study to demonstrate the feasibility and safety of a PCSEMS in patients with anastomotic stricture after LAG.
Overall, PCSEMS placement could be a feasible and effective option for management of benign anastomotic strictures after LAG and it appears to be safe in most patients. Further studies should be conducted to obtain more detailed information on the efficacy, safety, and long-term results of PCSEMS placement, and a comparative study comparing PCSEMS placement with other modalities (e.g., balloon dilation and bougination) for management of anastomotic stricture after LAG is also needed.
Abbreviations
- LAG:
-
Laparoscopy-assisted gastrectomy
- PCSEMS:
-
Partially covered self-expandable metallic stent
- GOOSS:
-
Gastric outlet obstruction scoring system
References
Carboni F, Lepiane P, Santoro R, Mancini P, Lorusso R, Santoro E. Laparoscopic surgery for gastric cancer: preliminary experience. Gastric Cancer. 2005;8:75–7.
Yano H, Monden T, Kinuta M, Nakano Y, Tono T, Matsui S, et al. The usefulness of laparoscopy-assisted distal gastrectomy in comparison with that of open distal gastrectomy for early gastric cancer. Gastric Cancer. 2001;4:93–7.
Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–7.
Park KK. Laparoscopic surgery for gastric cancer. Korean J Gastroenterol. 2005;45:9–16.
Topal B, Leys E, Ectors N, Aerts R, Penninckx F. Determinants of complications and adequacy of surgical resection in laparoscopic versus open total gastrectomy for adenocarcinoma. Surg Endosc. 2008;22:980–4.
Lee YC, Wang HP, Yang CS, Yang TH, Chen JH, Lin CC, et al. Endoscopic hemostasis of a bleeding marginal ulcer: hemoclipping or dual therapy with epinephrine injection and heater probe thermocoagulation. J Gastroenterol Hepatol. 2002;17:1220–5.
Lee SW, Nomura E, Bouras G, Tokuhara T, Tsunemi S, Tanigawa N. Long-term oncologic outcomes from laparoscopic gastrectomy for gastric cancer: a single-center experience of 601 consecutive resections. J Am Coll Surg. 2010;211:33–40.
Nagasako Y, Satoh S, Isogaki J, Inaba K, Taniguchi K, Uyama I. Impact of anastomotic complications on outcome after laparoscopic gastrectomy for early gastric cancer. Br J Surg. 2012;99:849–54.
Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68–72.
Schirmer BD. Current status of proximal gastric vagotomy. Ann Surg. 1989;209:131–48.
Ukleja A, Afonso BB, Pimentel R, Szomstein S, Rosenthal R. Outcome of endoscopic balloon dilation of strictures after laparoscopic gastric bypass. Surg Endosc. 2008;22:1746–50.
Peifer KJ, Shiels AJ, Azar R, Rivera RE, Eagon JC, Jonnalagadda S. Successful endoscopic management of gastrojejunal anastomotic strictures after Roux-en-Y gastric bypass. Gastrointest Endosc. 2007;66:248–52.
Tal AO, Friedrich-Rust M, Bechstein WO, Woeste G, Trojan J, Zeuzem S, et al. Self-expandable metal stent for malignant colonic obstruction: outcome in proximal vs. left sided tumor localization. Z Gastroenterol. 2013;51:551–7.
Lamazza A, Fiori E, Schillaci A, DeMasi E, Pontone S, Sterpetti AV. Self-expandable metallic stents in patients with stage IV obstructing colorectal cancer. World J Surg. 2012;36:2931–6.
No JH, Kim SW, Lim CH, Kim JS, Cho YK, Park JM, et al. Long-term outcome of palliative therapy for gastric outlet obstruction caused by unresectable gastric cancer in patients with good performance status: endoscopic stenting versus surgery. Gastrointest Endosc. 2013;78:55–62.
Fierz FC, Kistler W, Stenz V, Gubler C. Treatment of esophageal variceal hemorrhage with self-expanding metal stents as a rescue maneuver in a Swiss multicentric cohort. Case Rep Gastroenterol. 2013;7:97–105.
Holster IL, Kuipers EJ, van Buuren HR, Spaander MC, Tjwa ET. Self-expandable metal stents as definitive treatment for esophageal variceal bleeding. Endoscopy. 2013;45:485–8.
Swinnen J, Eisendrath P, Rigaux J, Kahegeshe L, Lemmers A, Le Moine O, et al. Self-expandable metal stents for the treatment of benign upper GI leaks and perforations. Gastrointest Endosc. 2011;73:890–9.
Loras C, Perez-Roldan F, Gornals JB, Barrio J, Igea F, Gonzalez-Huix F, et al. Endoscopic treatment with self-expanding metal stents for Crohn’s disease strictures. Aliment Pharmacol Ther. 2012;36:833–9.
Sandha GS, Marcon NE. Expandable metal stents for benign esophageal obstruction. Gastrointest Endosc Clin N Am. 1999;9:437–46.
Wadhwa RP, Kozarek RA, France RE, Brandabur JJ, Gluck M, Low DE, et al. Use of self-expandable metallic stents in benign GI diseases. Gastrointest Endosc. 2003;58:207–12.
Eubanks S, Edwards CA, Fearing NM, Ramaswamy A, de Torre la RA, Thaler KJ, et al. Use of endoscopic stents to treat anastomotic complications after bariatric surgery. J Am Coll Surg. 2008;206:935–8 (discussion 8–9).
Forshaw MJ, Sankararajah D, Stewart M, Parker MC. Self-expanding metallic stents in the treatment of benign colorectal disease: indications and outcomes. Colorectal Dis. 2006;8:102–11.
Lamazza A, Fiori E, De Masi E, Scoglio D, Sterpetti AV, Lezoche E. Self-expanding metal stents for treatment of anastomotic complications after colorectal resection. Endoscopy. 2013;45:493–5.
Adler DG, Baron TH. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. Am J Gastroenterol. 2002;97:72–8.
Manta R, Magno L, Conigliaro R, Caruso A, Bertani H, Manno M, et al. Endoscopic repair of post-surgical gastrointestinal complications. Dig Liver Dis. 2013;45(11):879–85.
Kim JH, Shin JH, Bae JI, Di ZH, Lim JO, Kim TH, et al. Gastric outlet obstruction caused by benign anastomotic stricture: treatment by fluoroscopically guided balloon dilation. J Vasc Interv Radiol. 2005;16:699–704.
Kim JH, Song HY, Park SW, Yoon CJ, Shin JH, Yook JH, et al. Early symptomatic strictures after gastric surgery: palliation with balloon dilation and stent placement. J Vasc Interv Radiol. 2008;19:565–70.
Tokunaga Y, Ryo J, Kitaoka A, Yagi T, Tokuka A, Ohsumi K. Jejunal pouch to avoid stricture after esophagojejunostomy with circular stapler. J Am Coll Surg. 1999;189:466–9.
Lee SE, Ryu KW, Nam BH, Lee JH, Kim YW, Yu JS, et al. Technical feasibility and safety of laparoscopy-assisted total gastrectomy in gastric cancer: a comparative study with laparoscopy-assisted distal gastrectomy. J Surg Oncol. 2009;100:392–5.
Conflict of interest
Kwang Hyun Chung, Sang Hyub Lee, Jin Myung Park, Jae Min Lee, Cheol Min Shin, Sang Hoon Ahn, Do Joong Park, Hyung-Ho Kim, Ji Kon Ryu, and Yong-Tae Kim declare that they have no conflict of interest or financial ties.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chung, K.H., Lee, S.H., Park, J.M. et al. Partially covered self-expandable metallic stent for postoperative benign strictures associated with laparoscopy-assisted gastrectomy. Gastric Cancer 19, 280–286 (2016). https://doi.org/10.1007/s10120-014-0450-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-014-0450-3