Abstract
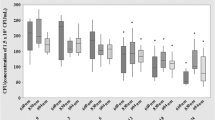
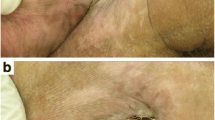
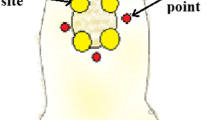
The aim of this study was to evaluate the effects of photobiomodulation (PBM) by dual-wavelength low-power lasers on the healing and bacterial bioburden of pressure ulcer (PU) models. Twenty-five male Swiss mice were divided into five equal groups. Ischemia reperfusion cycles were employed to cause PU formation by the external application of magnetic plates. Immediately after wounding, a suspension of Pantoea agglomerans was applied at the base of all the wounds of the infected groups, using a calibrated pipette. PBM (simultaneous emission at 660 and 808 nm, 142.8 J/cm2, in continuous wave emission mode) was applied to the PUs for 14 sessions. The animals were euthanized 14 days after PU induction, and their tissues were analyzed for wound contraction and reepithelialization, epidermis thickness, bacterial survival, and IL-1β and IL-10 mRNA level evaluations. The PU areas appeared larger in the mice from the infected groups than in those in the laser group 4 days after PU induction and presented incomplete reepithelialization 14 days after PU induction. However, the PBM accelerated the wound healing in the infected + laser group compared with the infected group 11 and 14 days following the PU induction. The infected and irradiated PUs exhibited a thinner neo-epidermis than those in the infected group, and the bacterial survival decreased in the laser group; the relative expression IL-1β mRNA levels demonstrated an increasing tendency while the relative expression IL-10 mRNA levels demonstrated a decreasing tendency in the infected + laser and laser groups. These results suggest that PBM improves healing by killing or inhibiting bacteria in PUs as well as by accelerating the wound healing, resulting in tissue repair.





Similar content being viewed by others
Change history
18 November 2019
The author name Maria Maria Côrtes Thomé Lima was incorrectly captured in the original article. The correct author name should be Andrezza Maria Côrtes Thomé Lima. The original article has been corrected.
References
Ammons MCB, Morrissey K, Tripet BP, Leuven JTV, Han A, Lazarus GS et al (2015) Biochemical association of metabolic profile and microbiome in chronic pressure ulcer wounds. PLoS One 10(5):e0126735
Rhoads DD, Wolcott RD, Sun Y, Dowd SE (2012) Comparison of culture and molecular identification of bacteria in chronic wounds. Int J Mol Sci 13(3):2535–2550
Stojadinovic O, Minkiewicz J, Sawaya A, Bourne JW, Torzilli P, de Rivero Vaccari JP et al (2013) Deep tissue injury in development of pressure ulcers: a decrease of inflammasome activation and changes in human skin morphology in response to aging and mechanical load. PLoS One 8(8):e69223
Coletti D, Teodori L, Lin Z, Beranudin JF, Adamo S (2013) Restoration versus reconstruction: cellular mechanisms of skin, nerve and muscle regeneration compared. Regen Med Res 1(1):4
Edsberg LE, Black JM, Goldberg M, McNichol L, Moore L, Sieggreen M (2016) Revised national pressure ulcer advisory panel pressure injury staging system: revised pressure injury staging system. J Wound Ostomy Continence Nurs 43(6):585–597
Rahim K, Saleha S, Zhu X, Huo L, Basit A, Franco OL (2017) Bacterial contribution in chronicity of wounds. Microb Ecol 73(3):710–721
Guo S, DiPietro LA (2010) Factors affecting wound healing. J Dent Res 89(3):219–229
Seth AK, Geringer MR, Nguyen KT, Agnew SP, Dumanian Z, Galiano RD et al (2013) Bacteriophage therapy for Staphylococcus aureus biofilm-infected wounds: a new approach to chronic wound care. Plast Reconstr Surg 131(2):225–234
Hamblin MR (2016) Photobiomodulation or low-level laser therapy. J Biophotonics 9(11–12):1122–1124
Avci P, Gupta A, Sadasivam M et al (2013) Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg 32(1):41–52
Hamblin MR (2017) Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys 4(3):337–361
Hamblin MR, Sousa MVP, Agrawal T (2017) Handbook of low-level laser therapy. Pan Stanford Publishing, Singapore
Freitas LF, Hamblin MR (2016) Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 22(3):7000417
Huang YY, Sharma SK, Carroll J, Hamblin MR (2011) Biphasic dose response in low level light therapy - an update. Dose-Response 9(4):602–618
Fekrazad R, Asefi S, Eslaminejad MB, Taghiar L, Bordbar S, Hamblin MR (2018) Photobiomodulation with single and combination laser wavelengths on bone marrow mesenchymal stem cells: proliferation and differentiation to bone or cartilage. Lasers Med Sci 27:1–12
Solmaz H, Ulgen Y, Gulsoy M (2017) Photobiomodulation of wound healing via visible and infrared laser irradiation. Lasers Med Sci 32(4):903–910
Silva DCGG, Plapler H, da Costa MM, Silva SRG, de Sá MCA, Silva BSL (2013) Low level laser therapy (AlGaInP) applied at 5J/cm2 reduces the proliferation of Staphylococcus aureus MRSA in infected wounds and intact skin of rats. An Bras Dermatol 88(1):50–55
Thomé AMC, Souza BP, Mendes JPM, Soares LC, Trajano ETL, Fonseca AS (2017) Dichromatic and monochromatic laser radiation effects on survival and morphology of Pantoea agglomerans. Laser Phys 27(5):055602
Thomé AMC, Souza BP, Mendes JPM, Cardoso AFR, Soares LC, Trajano ETL et al (2018) Dichromatic and monochromatic laser radiation effects on antibiotic resistance, biofilm formation, and division rate of Pantoea agglomerans. Laser Phys 28(6):065606
De Lima FJC, Barbosa FT, de Sousa-Rodrigues CF (2014) Use alone or in combination of red and infrared laser in skin wounds. J Lasers Med 5(2):51–57
Brito TLA, Monte-Alto-Costa A, Romana-Souza B (2014) Propranolol impairs the closure of pressure ulcers in mice. Life Sci 100:138–146
Nussbaum EL (2014) Effects of low intensity laser irradiation during healing of infected skin wounds in the rat. Photonics Lasers Med 3(1):23–36
Thomé AMC, Francisco NLSG, Amaral JPV, Soares LC, Trajano EDL (2018) Isolamento de bactérias de úlceras por pressão de pacientes internados em hospital universitário. Revista Pró-UniverSUS 09(1):46–50
Nascimento AP, Monte-Alto-Costa A (2006) Overweight induced by high-fat diet delays rat cutaneous wound healing. Br J Nutr 96:1069–1077
Trajano ET, Trajano LA, Dos Santos Silva MA, Venter NG, de Porto LC, de Fonseca A et al (2015) Low-level red laser improves healing of second-degree burn when applied during proliferative phase. Lasers Med Sci 30(4):1297–1304
Teixeira AF, Machado YLRC, Fonseca AS, Mencalha AL (2016) Low-level lasers and mRNA levels of reference genes used in Escherichia coli. Laser Phys Lett 13(11):115602 (6pp)
Turabelidze A, Guo S, DiPietro LA (2010) Importance of housekeeping gene selection for accurate reverse transcription-quantitative polymerase chain reaction in a wound healing model. Wound Repair Regen 18(5):460–466
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25(4):402–408
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453(7193):314–321
Takeo M, Lee W, Ito M (2015) Wound healing and skin regeneration. Cold Spring Harb Perspect Med 5(1):a023267
Ebright JR (2005) Microbiology of chronic leg and pressure ulcers: clinical significance and implications for treatment. Nurs Clin North Am 40(2):207–216
International Wound Infection Institute (IWII) (2016) Wound infection in clinical practice. Wounds International
Ashrafi M, Novak-Frazer L, Bates M, Baguneid M, Alonso-Rasgado T, Xia G et al (2018) Validation of biofilm formation on human skin wound models and demonstration of clinically translatable bacteria-specific volatile signatures. Sci Rep 8(1):9431
Rodrigo SM, Cunha A, Pozza DH, Blaya DS, Moraes JF, Weber JB et al (2009) Analysis of the systemic effect of red and infrared laser therapy on wound repair. Photomed Laser Surg 27(6):929–935
MacLeod AS, Mansbridge JN (2016) The innate immune system in acute and chronic wounds. Adv Wound Care (New Rochelle) 5(2):65–78
Jiang L, Dai Y, Cui F, Pan Y, Zhang H, Xiao J et al (2014) Expression of cytokines, growth factors and apoptosis-related signal molecules in chronic pressure ulcer wounds healing. Spinal Cord 52(2):145–151
Gohel MS, Windhaber RA, Tarlton JF, Whyman MR, Poskitt KR (2008) The relationship between cytokine concentrations and wound healing in chronic venous ulceration. J Vasc Surg 48(5):1272–1277
Ziraldo C, Solovyev A, Allegretti A, Krishnan S, Henzel MK, Sowa GA et al (2015) A computational, tissue-realistic model of pressure ulcer formation in individuals with spinal cord injury. PLoS Comput Biol 11(6):e1004309
Zhevago NA, Samoilova KA (2006) Pro- and anti-inflammatory cytokine content in human peripheral blood alter its transcutaneous (in vivo) and direct (in vitro) irradiation with polychromatic visible and infrared light. Photomed Laser Surg 24:129–139
Niemz MH (2007) Laser-tissue interactions: fundamentals and applications. Springer-Verlag, New York
Karu TI (2003) Low-power laser therapy. In: Vo Dinh T (ed) Biomedical photonics handbook. CRC Press, Boca Raton
Yan C, Gao N, Sun H, Yin J, Lee P, Zhou L, Fan X, Yu FS (2016) Targeting imbalance between IL-1β and IL-1 receptor antagonist ameliorates delayed epithelium wound healing in diabetic mouse corneas. Am J Pathol 186(6):1466–1480
Jetten N, Roumans N, Gijbels MJ et al (2014) Wound administration of M2-polarized macrophages does not improve murine cutaneous healing responses. PLoS One 9(7):e102994
Kurose T, Hashimoto M, Ozawa J, Kawamata S (2015) Analysis of gene expression in experimental pressure ulcers in the rat with special reference to inflammatory cytokines. PLoS One 10(7):e0132622
Funding
This study was funded by Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Universidade do Estado do Rio de Janeiro (UERJ), and Universidade Severino Sombra (USS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures were carried out in accordance with the Brazilian Legislation (no. 11.794, from October 8th, 2008) and were approved by the Ethical Committee for Animal Use of Universidade Severino Sombra (CEUA/007/2015).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The author name Maria Maria Côrtes Thomé Lima was incorrectly captured in the original article. The correct author name should be Andrezza Maria Côrtes Thomé Lima.
Rights and permissions
About this article
Cite this article
Thomé Lima, A.M.C., da Silva Sergio, L.P., da Silva Neto Trajano, L.A. et al. Photobiomodulation by dual-wavelength low-power laser effects on infected pressure ulcers. Lasers Med Sci 35, 651–660 (2020). https://doi.org/10.1007/s10103-019-02862-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-019-02862-w