Abstract
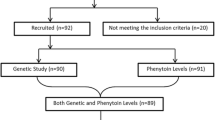
Phenytoin has a widespread use in epilepsy treatment and is mainly metabolized by hepatic cytochrome P450 enzymes (CYP). We have investigated CYP2C9*2, CYP2C9*3, CYP2C19*2 and CYP2C19*3 allelic variants in a Turkish population of patients on phenytoin therapy. Patients on phenytoin therapy (n = 102) for the prevention of epileptic seizures were included. Polymorphic alleles were analyzed by restriction fragment length polymorphism method. Serum concentrations of phenytoin were measured by fluorescence polarization immune assay method. The most frequent genotype was detected for CYP2C9 wild-type alleles (78.43 %), whereas CYP2C19*2/*2 (5.88 %) was the least frequent genotype group. According to the classification made with both enzyme polymorphisms, CYP2C9*1/*1-CYP2C19*1/*1 (G1: 41.17 %) genotype group was the most frequent whereas CYP2C9*1/*2-CYP2C19*1/*3 (G7: 0.98 %) was the least frequent one. The highest mean phenytoin level (27.95 ± 1.85 µg/ml) was detected in the G8 genotype group (CYP2C9*1/*3-CYP2C19*2/*3) and the G1 genotype group showed the lowest mean phenytoin level (7.43 ± 0.73 µg/ml). The mean serum concentration of phenytoin of the polymorphic patients with epilepsy was higher than that for the wild-type alleles both in the monotherapy and polytherapy patients. These results show the importance of the genetic polymorphism analysis of the main metabolizing enzyme groups of phenytoin for the dose adjustment.

Similar content being viewed by others
References
Ulrich K (2007) The role of pharmacogenetics in the metabolism of antiepileptic drugs pharmacokinetic and therapeutic implications. Clin Pharmocokinetic 46(4):271–279
Perucca E (1996) The new generation antiepileptic drugs: advantages and disadvantages. Br J Clin Pharmacol 42(5):531–543
Perucca E (2002) Patient-tailored antiepileptic drug therapy: predicting response to antiepileptic drugs. Int Congr Ser 1244:93–103
McNamara JO (2001) Drug effective in the therapy of the epilepsies. In: Hardman JG, Limbırd LE, Gilman AG (eds) Goodman and Gilman’s the pharmacological basis of therapeutics, 10th edn. McGraw-Hill, USA, pp 521–545
Patsalos PN, Berry DJ, Bourgeois BFD, Cloyd JC, Glauser TA et al (2008) Antiepileptic drugs—best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia 49:1239–1276
Yamazaki H, Asahi S, Gillam EMJ, Guengerich FP, Nakajima M et al (2000) Formation of a dihydroxy metabolite of phenytoin by human liver microsomes 7 cytosol: roles of cytochrome P450 2C9, 2C19 and 3A4. Drug Metab Dispos 28:1361–1368
Miners JO, Birkett DJ (1998) Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol 45:525–538
Ninomiya H, Mamiya K, Matsuo S, Ieiri I, Higuchi S et al (2000) Genetic polymorphism of the CYP2C subfamily and excessive serum phenytoin concentration with central nervous system intoxication. Ther Drug Monit 22:230–232
Mamiya K, Ieiri I, Shimamoto J, Yukawa E, Imaı J et al (1998) The effects of genetic polymorphisms of CYP2C9 and CYP2C19 on phenytoin metabolism in Japanese adult patients with epilepsy: studies in stereoselective hydroxylation and population pharmacokinetics. Epilepsia 39:1317–1323
Gestaut H (1969) Classification of the epilepsies: Proposal for an international classification. Epilepsia 10:14–21
Hauser WA, Kurland LT (1975) The epidemiology of epilepsy in Rochester, Minnesota 1935 through 1967. Epilepsia 16:1–66
Hung CC, Lin CJ, Chen CC, Chang CJ, Liou HH (2004) Dosage recommendation of phenytoin for patients with epilepsy with different CYP2C9/CYP2C19 polymorphisms. Ther Drug Monit 26(5):534–540
Yukawa E, Mamiya K (2006) Effect of CYP2C19 genetic polymorphism on pharmacokinetics of phenytoin and phenobarbital in Japanese epileptic patients using non-linear mixed effects model approach. J Clin Pharm Ther 31(3):275–282
Sanford JC, Guo Y, Sadee W, Wang D (2013) Regulatory polymorphisms in CYP2C19 affecting hepatic expression. Drug Metab Drug Interact 28(1):23–30
Aynacıoğlu AŞ, Brockmöller J, Bauer S, Güzelbey P, Öngen Z et al (1999) Frequency of cytochrome P 450 CYP2C9 variants in Turkish population and functional relevance for phenytoin. Br J Clin Pharmacol 48:409–415
Imai J, Ieiri I, Mamiya K, Miyahara S, Furuumi H et al (2000) Polymorphism of the cytochrome P450(CYP)2C9 gene in Japanese epileptic patients: genetic analysis of the CYP2C9 locus. Pharmacogenetics 10:85–89
Takanashi K, Tainaka H, Kobayashi K, Yasumori T, Hosakawa M et al (2000) CYP2C9 Ile359 and Leu359 variants: enzyme kinetic study with substrates. Pharmacogenetics 10:95–104
Dickmann U, Rettie AE, Kneller MB, Kim RB, Wood AJ et al (2001) Identification and functional characterization of a new CYP2C9 variant (CYP2C9*5) expressed among African-Americans. Mol Pharmacol 60:382–387
Robert SK, Timothy B, Curry SG, Timi E, Joyse B et al (2001) Identification of null allele of CYP2C9 in an African-American exhibiting toxicity to phenytoin. Pharmacogenetics 11:803–808
Weide J, Linda S, Steijns W, Marga JM, Keimpe H (2001) The effect of genetic polymorphism of cytocrom P450 CYP2C9 on phenytoin dose requirement. Pharmacogenetics 11:287–291
Blaisdell J, Lucia F, Nebert J, Coulter S, Ferguson SS et al (2004) Discovery of new potentially defective alleles of human CYP2C9. Pharmacogenetics 14:527–537
Basci NE, Bozkurt A, Kortunay S, Isimer A, Sayal A, Kayaalp SO (1993) Proguanil metabolism in relation to S- mephenytoin oxidation in a Turkish population. Br J Clin Pharmacol 42:771–773
Odani A, Hashimoto Y, Otsuki Y, Uwaı Y, Hattori H et al (1997) Genetic polymorphism of the CYP2C subfamily and its effect on the pharmacokinetics of phenytoin in Japanese patients with epilepsy. Clin Pharmacol Ther 62(3):287–292
Gaedigk A, Casley WL, Tyndale RF, Sellers EM, Jurima-Romet M et al (2001) Cytocrome P4502C9 (CYP2C9) allele frequencies in Canadian Native Indian and Inuit populations. Can J Physiol Pharmacol 79:841–847
Ingelman M, Sundberg K (2004) Pharmacogenetics of cytocrome P450 and applications in drug therapy the past, present and future. TRENDS Pharmacol Sci 25(4)
Argikar UA, Cloyd JC, Birnbaum AK, Leppik IE, Conway J et al (2006) Paradoxical urinary phenytoin metabolite (S)/(R) ratios in CYP2C19 *1/*2. Epilepsy Res 71:54–63
Yasumori T, Chen LS, Li QH, Ueda M, Tsuzukı Goldstein A et al (1999) Human CYP2C-mediated stereoselective phenytoin hydroxylation in Japanese: difference in chiral preference of CYP2C9 and CYP2C19. Biochem Pharmacol 57:1297–1303
Kerb R, Aynacioglu AS, Brockmöller J, Schlagenhaufer R, Bauer S et al (2001) The predictive value of MDR1, CYP2C9, and CYP2C19 polymorphisms for phenytoin plasma levels. Pharmacogenomics J 1(3):204–210
Scordo MG, Caputi AP, D’Arrigo C, Fava G, Spina E (2004) Allele and genotype frequencies of CYP2C9, CYP2C19 and CYP2D6 in an Italian population. Pharm Res 50:195–204
Griskevicius L, Yasar Ü, Sandberg M, Hidestrand M, Eliasson E et al (2003) Bioactivation cyclophosphamide: the role of polymorphic CYP2C enzymes. Eur J Clin Pharmacol 59:103–109
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Ozkaynakci and M. I. Gulcebi contributed to this study equally.
Rights and permissions
About this article
Cite this article
Ozkaynakci, A., Gulcebi, M.I., Ergeç, D. et al. The effect of polymorphic metabolism enzymes on serum phenytoin level. Neurol Sci 36, 397–401 (2015). https://doi.org/10.1007/s10072-014-1961-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-014-1961-8