Abstract
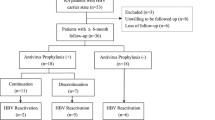
Hepatitis B virus (HBV) reactivation is a common complication of immunosuppressive treatment in high prevalence countries. Biological disease-modifying antirheumatic drugs (bDMARDs) cause this adverse event more often than conventional immunosuppressants. The incidence of HBV reactivation during treatment for rheumatic diseases in Germany is unclear. Furthermore, it remains open how to treat and monitor patients at risk during immunosuppressive therapy with bDMARDs. We examined 2054 patients from a German tertiary rheumatology center in order to analyze the prevalence of HBc-antibody-positivity and the incidence of HBV reactivation in German rheumatology patients treated with immunosuppressants. Of 1317 patients treated with bDMARDs and 737 conventional synthetic DMARD (csDMARDs) patients between 2008 and 2017, 86 had a history of HBV infection (anti-HBc positive). Only two patients were suffering from chronic infection (HBsAg positive). Three patients were treated pre-emptively with entecavir, and eight patients after HBV DNA reappearance. No liver failure occurred due to HBV reactivation. Compared to anti-HBc-positive patients without reactivation, the reactivation group included more patients exposed to three or more classes of bDMARDs (p = 0.017). The median HBs antibody titer was significantly lower in the reactivation group (15.0 IU/l vs. 293.5 IU/l; p = 0.001). This study shows that bDMARDs and csDMARDs can safely be administered to patients with a history of HBV, provided they are closely monitored. Low titers of anti-HBs antibodies and a history of ≥ 3 classes of immunosuppressants increase the risk of HBV reactivation. These data highlight major differences to high prevalence regions.

Similar content being viewed by others
References
Lai CL, Ratziu V, Yuen MF, Paynard T (2003) Viral hepatitis B. Lancet 362(9401):2089–2094
Trépo C, Chan HL, Lok A (2014) Hepatitis B virus infection. Lancet 384(9959):2053–2063
MacLachlan JH, Locarnini S, Cowie BC (2015) Estimating the global prevalence of hepatitis B. Lancet 386(10003):17–23 1515–1517
Cornberg M, Protzer U, Petersen J, Wedemeyer H, Berg T, Jilg W (2011) Prophylaxis, diagnosis and therapy of hepatitis B virus infection—the German guideline. Z Gastroenterol 49(7):871–930
European Association for the Study of the Liver (2017) EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 67:370–398
Lok ASF, McMahon BJ (2009) AASLD practice guideline update: chronic hepatitis B: update 2009. Hepatology 50(3)
Smolen JS, Keystone EC, Emery P, Breedveld FC, Betteridge N, Burmester GR, Dougados M, Ferraccioli G, Jaeger U, Klareskog L, Kvien TK, Martin-Mola E, Pavelka K, Working Group on the Rituximab Consensus Statement (2007) Consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis 66:143–150
Buch MH, Smolen JS, Betteridge N, Breedveld FC, Burmester G, Dörner T et al (2011) Updated consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis 70:909–920
Tang Z, Li X, Wu S, Liu Y, Qiao Y, Xu D, Li J (2017 Sep) Risk of hepatitis B reactivation in HBsAg-negative/HBcAb-positive patients with undetectable serum HBV DNA after treatment with rituximab for lymphoma: a meta-analysis. Hepatol Int 11(5):429–433
Singh JA, Saag KG, Bridges JRL, Akl EA, Bannuru RR, Sullivan MC et al (2016) 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 68(1):1–26
Pérez-Alvarez R, Díaz-Lagares C, García-Hernández F, Lopez-Roses L, Brito-Zerón P, Pérez-de-Lis M et al (2011) Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy. Medicine 90(6)
Barone M, Notarnicola A, Lopalco G, Viggiani MT, Sebastiani F, Covelli M, Iannone F, Avolio AW, di Leo A, Cantarini L, Lapadula G (2015) Safety of long-term biologic therapy in rheumatologic patients with a previously resolved hepatitis B viral infection. Hepatology 62:40–46
Mozessohn L, Chan KK, Feld JJ, Hicks LK (2015) Hepatitis B reactivation in HBsAg negative/HBcAb-positive patients receiving rituximab for lymphoma: a meta-analysis. J Viral Hepat 22:842–849
Ostuni P, Botsios C, Punzi L, Sfriso P, Todesco S (2003) Hepatitis B reactivation in a chronic hepatitis B surface antigen carrier with rheumatoid arthritis treated with infliximab and low dose methotrexate. Ann Rheum Dis 62:686–687
Lee YH, Bae SC, Song GG (2013) Hepatitis B virus (HBV) reactivation in rheumatic patients with hepatitis core antigen (HBV occult carriers) undergoing anti-tumor necrosis factor therapy. Clin Exp Rheumatol 31(1):118–121
Tien YC, Yen HH, Chiu YM (2017) Incidence and clinical characteristics of hepatitis B virus reactivation in HBsAg-negative/HBcAb-positive patients receiving rituximab for rheumatoid arthritis. Clin Exp Rheumatol 35(5):831–836
Perrillo RP, Gish R, Falck-Ytter YT (2015) American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 148(1):221–244.e3
Feuchtenberger M, Schaefer A, Nigg AP, Kraus MR (2016) Hepatitis B serology in patients with rheumatic diseases. Open Rheumatol J 10:39–48
Schmajuk G, Tonner C, Trupin L, Li J, Urmimala S, Ludwig D et al (2017) Using health-system-wide data to understand hepatitis B virus prophylaxis and reactivation outcomes in patients receiving rituximab. Medicine 96:13
Paul S, Shuja A, Tam I, Kim EM, Kang S, Kapulsky L, Viveiros K, Lee H (2016) Gastroenterologists have suboptimal hepatitis B virus screening rates in patients receiving immunosuppressive therapy. Dig Dis Sci 61(8):2236–2241
van der Have M, Belderbos TD, Fidder HH, Leenders M, Dijkstra G, Peters CP, Eshuis EJ, Ponsioen CY, Siersema PD, van Oijen M, Oldenburg B, Dutch Initiative on Crohn and Colitis (ICC) (2014) Screening prior to biological therapy in Crohn’s disease: adherence to guidelines and prevalence of infections. Results from a multicentre retrospective study. Dig Liver Dis 46(10):881–886
Loras C, Gisbert JP, Mínguez M, Merino O, Bujanda L, Saro C et al (2010) Liver dysfunction related to hepatitis B and C in patients with inflammatory bowel disease treated with immunosuppressive therapy. Gut 59(10):1340–1346
Carroll MB, Forgione MA (2010) Use of tumor necrosis factor α inhibitors in hepatitis B surface antigen-positive patients: a literature review and potential mechanisms of action. Clin Rheumatol 29:1021–1029
Gonzalez S, Perillo RP (2016) Hepatitis B virus reactivation in the setting of cancer chemotherapy and other immunosuppressive drug therapy. Clin Infect Dis 62(S4):S306–S313
Koo YX, Tay M, The YE, Teng D, DSW T, Tan IBH et al (2011) Risk of hepatitis B virus (HBV) reactivation in hepatitis B surface antigen negative/hepatitis core antibody positive patients receiving rituximab-containing combination chemotherapy without routine antiviral prophylaxis. Ann Hematol 90:1219–1223
Chen CY, Tien FM, Cheng A, Huang SY, Chou WC, Yao M et al (2018) Hepatitis B reactivation among 1962 patients with hematological malignancy in Taiwan. BMC Gastroenterol 18(1):6
Matsubara T, Nishida T, Shimoda A, Shimakoshi H, Amano T, Sugimoto A et al (2017) The combination of anti-HBc and anti-HBs levels is a useful predictor of the development of chemotherapy-induced reactivation in lymphoma patients with resolved HBV infection. Oncol Lett 14(6):6543–6552
Vassilopoulos D, Apostolopoulou A, Hadziyannis E, Papatheodoridis GV, Manolakopoulos S, Koskinas J, Manesis EK, Archimandritis AI (2010) Long-term safety of anti-TNF treatment in patients with rheumatic diseases and chronic or resolved hepatitis B virus infection. Ann Rheum Dis 69:1352–1355
Nazi I, Kelton JG, Larché M, Snider DP, Heddle NM, Crowther MA et al (2013) The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood 122:1946–1953
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E.C. Schwaneck, H-P. Tony, O. Gadeholt, and M. Schmalzing have received travel grants, speaker’s fees, research grants, or compensation for board membership and consultancies from Celgene AbbVie, Baxalta (Shire), Chugai, Roche, Janssen, Pfizer, MSD, UCB, Novartis, and Lilly. S. Kreissl-Kemmer received a travel grant from Daiichi Sankyo. M. Krone has no competing interests. J. Weiss has received travel grants, speaker’s fees, or compensation for board membership and consultancies from AbbVie, BMS, and Gilead. A. Geier has received travel grants, speaker’s fees, research grants, or compensation for board membership and consultancies from AbbVie, BMS, Gilead, Janssen, Falk, Sequana, and Novartis. B. Weissbrich has no competing interests.
Additional information
Key messages
• Patients with low anti-HBs antibodies have a higher risk for HBV reactivation.
• Multiple successive, immunosuppressive therapies result in a high risk of HBV reactivation.
• Most patients with a history of HBV can safely receive antirheumatic therapies.
Rights and permissions
About this article
Cite this article
Schwaneck, E.C., Krone, M., Kreissl-Kemmer, S. et al. Management of anti-HBc-positive patients with rheumatic diseases treated with disease-modifying antirheumatic drugs—a single-center analysis of 2054 patients. Clin Rheumatol 37, 2963–2970 (2018). https://doi.org/10.1007/s10067-018-4295-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-4295-8