Abstract
With the advent of coronavirus disease (COVID-19) pandemic, a wide range of life-threatening maxillofacial fungal coinfections have also been observed in patients. We conducted this systematic review to collate and evaluate the data to enable clinicians to understand the disease pattern and types of mycosis and provide meticulous management of these infections in COVID-19 patients. The review was carried out in accordance with Preferred Reporting Items for Systematic Review and Meta-analysis guidelines. A systematic electronic literature search was conducted on major databases using keywords in combination with Boolean Operators. Manuscripts discussing cases of maxillofacial fungal infections in COVID-19 patients were included. A total of 11 studies were systematically reviewed to assess the fungal coinfections in COVID-19 patients. Twenty-one cases of mucormycosis, 58 of candidiasis, and 1 each of aspergillosis and mixed infection were observed in the region of head and neck. Significant increase in invasive fungal infection is evident in patients suffering from COVID-19 which could be due to immunosuppression and other pre-existing comorbidities. Early diagnosis and intervention like systemic antifungals or surgical debridement is mandatory to reduce morbidity and mortality.
Similar content being viewed by others
Introduction
Healthcare systems worldwide, with the advent of new decade of 2020, are fighting a catastrophe due to coronavirus disease (COVID-19), an emerging infectious syndrome caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. In December 2019, Wuhan in China became the epicentre of this pandemic coronavirus disease 2019 (COVID-19) which has become a major challenging public health problem not only for China but also for the rest of the countries around the world. The World Health Organization (WHO) announced that this outbreak had constituted a public health emergency of international concern [2]. More than 150 million people have been afflicted worldwide by this disease till April 2021. India has reported 19.5 million cases till date with more than 215,542 patients succumbing to this disease (https://www.worldometers.info/coronavirus/country/india/).
This novel strain of SARS-CoV-2 apart from causing pneumonia is also reported to cause strokes, renal failure, cardiomyopathy, venous thrombosis, and vasculitis [3, 4]. Pneumonia is manifested in infected patients when the virus binds to Angiotensin Converting Enzyme 2 (ACE 2) receptors [5] COVID-19 patients have decreased CD4 + T and CD8 + T cells leading to immunosuppression [6] The second severe stage of this disease occurs due to systemic inflammation and cardiopathy causing hepatic, cardiac, or renal damages [6] Critically ill COVID-19 patients in intensive care units (ICU) or on mechanical ventilation are more prone to bacterial or fungal nosocomial infections [7, 8] A marked increase in the cases of invasive maxillofacial fungal infections like mucormycosis, candidiasis, and aspergillosis has been observed in such infected patients. It is pivotal to understand these secondary infections and their aetiology for optimal management of the patients.
Candida species like C. albicans, C. glabrata, C. tropicalis, and C. krusei are normal commensals inhabiting mucosal surfaces like skin, respiratory, urinary, or digestive tracts in humans. The patients who are immunocompromised or on long-term pharmacotherapies have a tendency to develop mucosal candidiasis. Oropharyngeal candidiasis (OPC) caused predominantly by colonisation of C. albicans can be a cause of morbidity in these patients [9] Mortality rate attributed to invasive candidiasis is 19–40%, which can increase to around 70% for ICU patients [10, 11] Mucormycosis is a rare fungal disease with rhino-orbital-cerebral being the most common type caused by inhalation of spores into paranasal sinus of susceptible individuals. Fatality rate of this fungal infection is 46% occurring due to vascular thrombosis, angioinvasion, and tissue necrosis [12] Aspergillosis is another fungal disease invading the sinuses of immunocompromised patients, caused by species Aspergillus fumigatus [13].
The multitudinous increase of these maxillofacial fungal infections in COVID-19 patients in the past 1 year has been a cause of concern. The hosts at substantial risk of developing these fungal infections could be diabetics, immunocompromised individuals, patients on corticosteroids, and those with hematologic insufficiencies [14] New cases are being diagnosed every day yet they are under-reported. In the present manuscript, we have systematically reviewed the cases of invasive fungal infections affecting maxillofacial region in patients who suffered from COVID-19 to enable clinicians and laboratory experts to understand the disease pattern and meticulous management of these comorbidities.
Methodology
The present systematic review was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Review and Meta-analysis) guidelines.
Search strategy
MEDLINE (accessed from PubMed), Cochrane Library, Scopus, and Google Scholar were systematically and extensively searched to identify published data on invasive maxillofacial fungal infections reported in COVID-19 patients. Search strategy was implemented using keywords such as ‘Maxillofacial’; ‘Oral’; ‘Head & Neck region’; ‘Fungal Infections’; ‘COVID 19’ in combination with Boolean Operators ‘AND’ and ‘OR’. All the manuscripts published in English language till the month of April 2021 were reviewed for inclusion in the study. Attempts were also made to screen grey literature and references of all the manuscripts to obtain additional data for the present review.
Inclusion and exclusion criteria
Manuscripts with information about fungal infections like candidiasis, mucormycosis, aspergillosis and others affecting the maxillofacia region of patients suffering from COVID-19 were included in the present review. Only case reports, case series, or retrospective studies reporting cases were included.
The studies which discussed about fungal infections of other locations, those with doubtful diagnosis, the studies reporting non-fungal infections, not relating to epidemiological or clinical interests of study or manuscripts in language apart from English were excluded.
Data extraction
Data from the studies was extracted by two reviewers independently. They screened all the titles and the abstracts obtained by applying the search strategies and inclusion criteria. Full manuscripts were obtained for the studies that appeared to meet inclusion criteria or in case of any uncertainty. Disagreement was resolved through discussions. A third reviewer was approached to resolve any divergence in opinions amongst the two main reviewers. Authors were not blind to journal titles or to authors or their institutions. The manuscripts included were reviewed for the type of fungal infections, site, gender, clinical presentation, and management. Though the quality assessment could not be done formally for all the studies, the findings were tabulated and synthesised narratively.
Risk of bias was evaluated using MINOR’s checklist for non-randomised studies. It was evaluated for two studies as the rest of the included studies were case reports.
Results
A total of 315 manuscripts were retrieved after the initial search using the abovementioned keywords, out of which 295 articles were rejected after screening the titles and abstracts. Full texts of a total of 20 articles were reviewed for inclusion in the review. Another nine studies were excluded because they either were reviews or discussed about infections of regions other than maxillofacial areas. Hence, a total of 11 studies were systematically reviewed [15,16,17,18,19,20,21,22,23,24,25]. The characteristics of included studies are depicted in Table 1. The results of the systematic literature search have been depicted in Fig. 1.
According to the data extracted from the final 11 manuscripts, invasive fungal infections have been reported in the COVID-19 patients in the maxillofacial region which included oral cavity along with rhino-orbito-cerebral complex. Out of the 81 cases reported, 21 cases were of mucormycosis, 58 were candidiasis, and 1 each of aspergillosis and mixed infection. It was observed that oral candidiasis was more common in the patients followed by mucormycosis of rhino-cerebro-orbital region. Aspergillosis and mixed fungal infection of orbital and maxillary region was observed. Age group affected by maxillofacial fungal infections ranged from 33 to 73 years with males being predominantly infected. Majority of the patients who developed maxillofacial fungal coinfections were either diabetic, suffering from cardiovascular diseases or on corticosteroids. Only one patient was reportedly suffering from hypothyroidism. The treatment provided to the patients included antifungals like Fluconazole (Zoltec® 200 mg/100 mL) or oral Nystatin (100,000 IU/mL, 8/8 h, for 30 days) [15, 17, 23, 25]. For the infections involving oral cavity, patients were advised to maintain good oral hygiene, use chlorhexidine digluconate (0.12%) mouth rinses, and apply 1% hydrogen peroxide daily.
Out of the studies included, 2 are non-randomised studies for which risk of bias is depicted in Table 2. The rest nine studies were case reports for which risk of bias could not be assessed. There is no significant risk of bias amongst the studies analysed.
Discussion
The novel strain of coronavirus of family Coronaviridae, order Nidovirales comprising large, single, plus stranded RNA as their genome has caused a worldwide pandemic burden along with life-threatening outcomes [26]. This virus has special abilities to dysregulate the host immune mechanisms. These impaired cell-mediated immune responses and overexpression of inflammatory cytokines cause enormous collateral damage increasing the morbidity and mortality associated with the COVID-19 pandemic. Also, the COVID-19 patients with immunocompromised states like neutropenia, inherited immunodeficiencies, HSCT, tumours, or long-term corticosteroid administration have increased likelihood of fungal coinfections [9]. Little is discussed in literature about these fungal infections damaging the maxillofacial regions and the oral cavities of COVID-19 patients, although such cases are multiplying every day. Sepsis associated with COVID-19 can damage the mucosal barriers and translocation of concentrated fungus in blood leading to fungemia [27], which can also prove to be lethal for the patients when it involves the maxillofacial region. Despite the minacious consequences, these fungal infections are often misdiagnosed or missed in COVID-19 patients. Hence, we conducted this systematic review to bring to light the kinds of fungal infections reported in COVID-19 patients, their clinical picture, associated risks, diagnostic methods, and treatment modalities. According to the present review, total 81 cases of mucomycosis, candidiasis, and aspergillosis of maxillofacial region have been reported in COVID-19 patients [15,16,17,18,19,20,21,22,23,24,25].
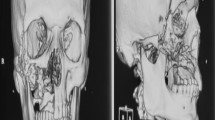
Mucormycosis is a rare and fatal deep fungal infection occurring in rare and aggressive forms caused by the family Mucoraceae which is difficult to diagnose [28]. This fungus is a usual commensal of nasal mucosa. The fungal spores germinate into hyphae on entering the tissues of hosts initiating clinical symptoms and causing infection in immunocompromised individuals with defective phagocytic functions [29] Impaired phagocytic function and increased hyphae levels cause thrombosis, ischemia, infarction, and tissue necrosis. Fungal spores, in patients with diabetic ketoacidosis, severe burns, tumours, organ transplants or those on corticosteroids, can germinate in nasal cavity, paranasal sinuses to palate, orbit and even the brain, leading to death sometimes [30] There are six clinical forms of mucormycosis, most common being the rhino-cerebro-orbital form (44–48%) followed by the cutaneous variety (10–19%), then pulmonary (10–11%), disseminated (6–10%) and last being the gastrointestinal form (2–11%) [31, 32] In the present review, we observed 21 cases of rhino-cerebro-orbital mucormycosis in COVID-19 patients. Majority of cases were reported in patients who had history of diabetes or were on corticosteroid therapy [16,17,18,19,20]. Incidence of mucomycosis is independent of age- or gender-related factors, though in this study a greater number of males were reportedly affected which could have been due to more prevalence of COVID-19 amongst males [17, 20] Signs and symptoms reported by majority of the patients included in this review were fever, headache, orbital cellulitis with palpebral edema, ptosis, ophthalmoplegia, unilateral facial swelling and swelling in premaxillary, malar and retrobulbar region [16,17,18,19,20]. Delay in treatment of mucormycosis for even a week doubles the mortality rate from 35 to 66% and makes the prognosis poorer [19].
Candidiasis is the most common type of superficial fungal infection. Candida species is a frequent inhabitant of oral mucosa, but its growth is inhibited by other organisms in the body that prevents any pathological alteration of mucosa by this fungus. Candida albicans is the most prevalent yeast species followed by Candida glabrata, Candida krusei, Candida tropicalis, and Candida stellatoidea [14]. According to this systematic review, 57 cases of oral candidiasis and one case of candida retinitis have been observed in patients undergoing treatment for COVID-19. Infection with coronavirus triggers an exaggerated immune response and inflammatory cascade, but the exact mechanism of the immune-mediated pathways leading to COVID-19 associated candidiasis is not very clear. Increased neutrophil-to-lymphocyte ratio, monocyte-derived macrophages, and reduced expression of human leukocyte antigen DR on the monocytes observed in severe cases of COVID-19 can be indirectly related to systemic candida infections [33, 34] Systemic medications such as antibiotics, immunosuppressants, and anti-cholinergic drugs, diabetes mellitus, nutritional disorders, endocrinopathies, malignancies, or decreased salivary antimicrobial proteins are amongst the classical risk factors that can cause invasive candidiasis in COVID-19 patients rather than just the disrupted immune mechanisms [35, 36] Pseudomembranous, hyperplastic, and erythematous are the three major forms of oral candidiasis. Majority of the patients in our study reported pseudomembranous kind of candidiasis presenting as yellowish-white scrapable plaques on oral mucosal surfaces, tongue and palate being the most affected sites. From this study we also observed that pseudomembranous candidiasis developed in patients in around 7–8 days of getting infected with coronavirus. It was also noticed that more cases occurred in elderly patients, those above 50 years of age. In our review, oral and retinal candidiasis was seen in COVID-19 patients who were either diabetic, having cardiovascular diseases, hypothyroidism, on corticosteroids or in ICU settings which is in accordance with other studies that reported fungal coinfections of other sites [3, 8, 37, 38].
Aspergillosis is an opportunistic infection being the second most common kind of oral and maxillofacial mycosis [39] The species that most frequently causes infection is Aspergillus fumigatus followed by Aspergillus niger, Aspergillus flavus, and Aspergillus terreus [40]. This fungus invades vascular tissues causing thrombosis and infarction. Aspergillosis can be invasive, destructive non-invasive and non-invasive. Invasive aspergillosis is characterised by invasion of the yeast into tissues with slowly progressive and destructive or highly aggressive and lethal infections [41]. Aspergillosis of maxillary sinus invades oral tissues causing palatal infarctions and also disseminating into systemic circulation at times. Immunocompromised individuals are at a higher risk of developing this infection. There was just one case of aspergillosis of maxillofacial region in COVID-19 patient reported who was an immunocompromised elderly female having diabetes and was on corticosteroid therapy [17].
Early diagnosis and prompt management is mandatory for these maxillofacial fungal coinfections to improve the prognosis and decrease the morbidity in patients suffering from COVID-19. Computed tomography (CT) is the main diagnostic tool to assess mucormycosis involving sinuses, and the extent of spread beyond sinus is confirmed by magnetic resonance imaging (MRI). Definitive diagnosis is achieved by histologic examination (non-septate hyphae that branch at 90 degree), culture and KOH examination [28] Aspergillosis of maxillofacial region is also diagnosed by histological examination which shows fungal hyphae branching at 45 degree, conidiospores and fruiting bodies [41] Definitive diagnosis for oral candidiasis can be made by exfoliative cytology, microbiological culture, potassium peroxide staining, salivary assay, or oral mucosal biopsy [42] Management of mucormycosis can be both medicinal and surgical. Liposomal Amphotericin B and oral fluconazole are used for prophylactic management against all fungal hyphae [20, 43]. Oral candidiasis can be treated using 10 mg clotrimazole tablets or oral nystatin suspension (400,000–600,000 IU/ml) as oral rinses [23, 25] Flucystine is administered for treatment of the systemic candidiasis [25]. Antifungal therapy of 200 mg of fluconazole tablets on the first day followed by 100–200 mg fluconazole daily for 7–14 days is provided for management of fungal coinfections [25, 42, 44]. Surgical management of mucormycosis includes resecting infected and necrotic tissues extensively to reduce the fungal load. Surgical debridement provides adequate tissues for biopsy also, to histopathologically confirm the diagnosis of fungal infection. Dissection is continued until normal perfused tissue is reached since bleeding from affected tissues is less likely because of thrombosis of vessels. In extensive cases of involvement of maxilla, palate, nasal cartilage, or orbit, additional surgeries may be required. Orbital decompression or exenteration can be done in cases of orbital involvement. Partial or total maxillectomy, craniectomy with cranial prosthesis insertion, ethmoidectomy are other treatment modalities which may cause significant disfigurement but are imperative in certain cases. Immediate reconstruction after deep resection is recommended to decrease exposure of vital tissues and tissue atrophy [45,46,47]. Similar treatment modalities for the management of 81 cases are assessed in our review. It was observed that nine patients of mucormycosis did not survive despite the therapy provided, due to cerebral oedema and other complications arising from fungal coinfections [17, 19, 20]. Cerebral oedema observed in the patient later evolved into multiple encapsulated fluid collections in bifrontal region which further complicated the case. Also, corticosteroids administered to mucormycosis patients suppressed their immunity making them prone to secondary infections. The limitation of the present study is that it includes only case reports and case series due to limited reporting of literature but rapidly increasing cases of fungal infections in COVID patients today are a matter of great concern. The high mortality rate of this disease makes early intervention by systemic antifungals, aggressive surgical debridement and management of underlying co-morbidities essential for survival of COVID-19 patients.
Conclusion
COVID-19 pandemic has caused a great havoc on health and social well-being of individuals worldwide since March 2020. From the present review, we summarise that COVID-19 can be associated with significance incidence of maxillofacial fungal coinfections which can prove to be life threatening. Clinicians must be aware of likelihood of this mycosis particularly in patients with pre-existing risk factors. All the patients should be closely monitored for immunosuppression sequelae following completion of treatment. It is pivotal to assess these risk factors, type of mycosis, and diagnostic methods and provide immediate management in to significantly reduce the morbidity and mortality occurring from these fungal coinfections.
References
Not applicable for disease control and prevention. Transmission of corona virus disease 2019 (COVID 19). Available at https://www.cdc.gov/coronavirus/2019-ncov/about-transmission.html
Mahase E (2020) China coronavirus: WHO declares international emergency as death toll exceeds 200. BMJ 368:m408
Guan WJ et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382(18):1708–1720. https://doi.org/10.1056/NEJMoa2002032 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Goyal P et al (2020) Clinical characteristics of Covid19 in New York City. N Engl J Med. https://doi.org/10.1056/nejmc2010419
Sweeney JM, Barouqa M, Krause GJ, Gonzalez-Lugo JD, Rahman S, Gil MR (2020) Evidence for secondary thrombotic microangiopathy in COVID-19. medRxiv. https://doi.org/10.1101/2020.10.20.20215608
Peman J, Gaitan AR, Vidal CG, Salavert M, Ramirez P, Puchades F, Hita MG, Izquierdo AA, Quindo G (2020) Fungal co-infection in COVID-19 patients: should we be concerned? Rev Iberoam Micol 37(2):41–46. https://doi.org/10.1016/j.riam.2020.07.001
Arastehfar A, Carvalho A, Van De Veerdonk FL, Jenks JD, Köhler P, Krause R, Cornely OA, Perlin DS, Lass-Flörl C, Hoenigl M (2020) COVID-19 associated pulmonary aspergillosis (CAPA)—from immunology to treatment. J Fungi 6:91
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395:507–513
Rolling T, Hohl TM, Zhai B (2020) Minority report: the intestinal mycobiota in systemic infections. Curr Opin Microbiol 56:1–6
Kullberg BJ, Arendrup MC (2015) Invasive candidiasis. New Engl. J. Med. 373:1445–1456 [CrossRef]
Marra AR, Camargo LFA, Pignatari ACC, Sukiennik T, Behar PRP, Medeiros EAS, Ribeiro J, Girão E, Correa L, Guerra C et al (2011) Nosocomial bloodstream infections in Brazilian hospitals: analysis of 2,563 cases from a prospective nationwide surveillance study. J Clin Microbiol 49:1866–1871
Cox G (2020) Mucormycosis. UpToDate (8)
International Diabetes Federation. [Jul;2020 ]; https://idf.org/our-network/regions-members/south-east-asia/members/94-india.html
Serris A, Danion F, Lanternier F (2019) Disease entities in mucormycosis. J Fungi 5(1):23
Bhagali R, Prabhudesai NP, Prabhudesai MN (2021) Post COVID-19 opportunistic candida retinitis: a case report. Indian J Ophthalmol 69(4):987–989
Dallalzadeh LO, Ozzello DJ, Liu CY, Kikkawa DO, Korn BS (2021) Secondary infection with rhino-orbital cerebral mucormycosis associated with COVID-19. Orbit:1–4
Moorthy A, Gaikwad R, Krishna S, Hegde R, Tripathi KK, Kale PG, Rao PS, Haldipur D, Bonanthaya K (2021) SARS-CoV-2, uncontrolled diabetes and corticosteroids-an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxillofac Oral Surg 20(3):1–8
Mekonnen ZK, Ashraf DC, Jankowski T, Grob SR, Vagefi MR, Kersten RC, Simko JP, Winn BJ (2021) Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthalmic Plast Reconstr Surg 37(2):e40–e80
Werthman-Ehrenreich A (2021) Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med 42:264.e5-264.e8
Mehta S, Pandey A (2020) Rhino-orbital mucormycosis associated with COVID-19. Cureus 12(9):e10726
Riad A, Gad A, Hockova B, Klugar M (2020) Oral candidiasis in non-severe COVID-19 patients: call for antibiotic stewardship. Oral Surg. https://doi.org/10.1111/ors.12561
Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, De Paula RM, Cembranel AC, Santos-Silva AR, Guerra ENS (2020) Oral mucosal lesions in a COVID-19 patient: new signs or secondary manifestations? Int J Infect Dis 97:326–328
Corchuelo J, Ulloa FC (2020) Oral manifestations in a patient with a history of asymptomatic COVID-19: case report. Int J Infect Dis 100:154–157
Díaz Rodríguez M, Jimenez Romera A, Villarroel M (2020) Oral manifestations associated with COVID-19. Oral Dis. https://doi.org/10.1111/odi.13555
Salehi M, Ahmadikia K, Mahmoudi S, Kalantari S, Jamalimoghadamsiahkali S, Izadi A, Kord M, Dehghan Manshadi SA, Seifi A, Ghiasvand F, Khajavirad N, Ebrahimi S, Koohfar A, Boekhout T, Khodavaisy S (2020) Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: species identification and antifungal susceptibility pattern. Mycoses 63(8):771–778
Fehr AR, Perlman S (2015) Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol 1282:1–23
Leelahavanichkul A, Worasilchai N, Wannalerdsakun S, Jutivorakool K, Somparn P, Issara-Amphorn J, Tachaboon S, Srisawat N, Finkelman M, Chindamporn A (2016) Gastrointestinal leakage detected by serum(1!3)-_-D-glucan in mouse models and a pilot study in patients with sepsis. Shock 46:506–518
Ferguson BJ (2000) Mucormycosis of the nose and paranasal sinuses. Otolaryngol Clin N Am 33(2):2000
Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP (2012) Pathogenesis of mucormycosis. Clin Infect Dis 54(suppl_1):S16–S22
Hingad N, Kumar G, Deshmukh R (2012) Oral mucormycosis causing necrotizing lesion in a diabetic patient: a case report. Int J Oral Maxillofac Pathol 3(3)
Muzyka BC, Epifanio RN (2013) Update on oral fungal infections. Dent Clin North Am 57(4):561–581
Arnaiz-García ME, Alonso-Peña D, Gonzalez-Vela MC, García-Palomo JD, RicoJR Sanz-Gimenez-, Arnáiz-García AM (2009) Cutaneous mucormycosis: report of five cases and review of the literature. J Plast Reconstr Aesthet Surg. 62:434e441
Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, Xu Y, Tian Z (2020) Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol 17:533–535 [CrossRef]
Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami M-E, Katsaounou P et al (2020) Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 27:992–1000
Mead G (2002) Management of oral mucositis associated with cancer chemotherapy. Lancet 359(9309):815–816. https://doi.org/10.1016/S0140-6736(02)07960-6
Ninane J (1994) Group MS A multicentre study of fluconazole versus oral polyenes in the prevention of fungal infection in children with hematological or oncological malignancies. Eur J Clin Microbiol Infect Dis 13(4):330–337
Gangneux J-P, Bougnoux M-E, Dannaoui E, Cornet M, Ralph ZJ (2020) Invasive fungal diseases during COVID-19: we should be prepared. J Mycol Med 30(2):100971
Laudenbach JM, Epstein JB (2009) Treatment strategies for oropharyngeal candidiasis. Expert Opin Pharmacother 10(9):1413–1421
Krishnan PA (2012) Fungal infections of the oral mucosa. Indian J Dental Res 23(5):650–659
Hartwick RW, Batsakis JG (1991) Sinus aspergillosis and allergic fungal sinusitis. Ann Otol Rhinol Laryngol 100(5 Pt 1):427–430
Deepa A, Nair BJ, Sivakumar T, Joseph AP (2014) Uncommon opportunistic fungal infections of oral cavity: a review. J Oral Maxillofac Pathol 18(2):235–243
Samaranayake LP, Keung Leung W, Jin L (2009) Oral mucosal fungal infections. Periodontology 2000 49(1):39–59
Morace G, Borghi E (2012) Invasive mold infections: virulence and pathogenesis of mucorales. Int J Microbiol 2012:349278
Rajendra Santosh AB, Muddana K, Bakki SR (2021) Fungal infections of oral cavity: diagnosis, management, and association with COVID-19. SN Compr Clin Med:1–12
Luo QL, Orcutt JC, Seifter LS (1989) Orbital mucormycosis with retinal and ciliary artery occlusions. Br J Ophthalmol 73(8):680–683
Kohn R, Hepler R (1985) Management of limited rhino-orbital mucormycosis without exenteration. Ophthalmology 92(10):1440–1444
Chander J, Kaur M, Singla N, Punia RPS, Singhal SK, Attri AK, Alastruey-Izquierdo A, Stchigel AM, Cano-Lira JF, Guarro J (2018) Mucormycosis: battle with the deadly enemy over a five-year period in India. J Fungi (Basel) 4(2):46
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable since it is a systematic review.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jain, A., Taneja, S. Post-COVID fungal infections of maxillofacial region: a systematic review. Oral Maxillofac Surg 26, 357–363 (2022). https://doi.org/10.1007/s10006-021-01010-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10006-021-01010-5