Abstract
Nanoporous graphene is being regarded as a promising candidate for reliable gas separation and purification applications. In the present research, the permeation barrier, selectivity and all thermodynamic functions for passing of four different molecules including CH4, H2S, N2 and CO2 gases on four types of porous graphene which is doped by two, three and six nitrogen atoms using quantum mechanical modelling, based on the density functional theory, B97D, and cc-pVTZ basis set have been evaluated. We find that the permeation barrier of all studied gases especially carbon dioxide decreased by considering the functionalized porous graphene by two, three and six nitrogens–doped, respectively. The results of our study propose using a porous graphene sheet as highly efficient and highly selective membranes for gas separations.
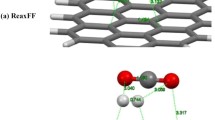
Graphical abstract










Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article.
References
Kumar R, Kumar A, Singh R et al (2020) Room temperature ammonia gas sensor using Meta Toluic acid functionalized graphene oxide. Mater Chem Phys 240:121922
Hosseingholipourasl A, Hafizah Syed Ariffin S, Al-Otaibi YD et al (2020) Analytical approach to study sensing properties of graphene based gas sensor. Sensors 20(5):1506
Demon SZ, Kamisan AI, Abdullah N et al (2020) Graphene-based materials in gas sensor applications: a review. Sens Mater 32(2):759–777
Pawar D, Kanawade R, Kumar A et al (2020) High-performance dual cavity-interferometric volatile gas sensor utilizing graphene/PMMA nanocomposite. Sensor Actuat B Chem :127921
Xu B, Huang J, Xu X et al (2019) Ultrasensitive NO gas sensor based on the graphene oxide-coated long-period fiber grating. ACS Appl Mater Interfaces 11(43):40868–40874
Szczęśniak B, Choma J (2020) Graphene-containing microporous composites for selective CO2 adsorption. Microporous Mesoporous Mater 292:109761
Nikkho S, Mirzaei M, Sabet JK et al (2020) Enhanced quality of transfer-free graphene membrane for He/CH4 separation. Sep Purif Technol 232:115972
Ali A, Aamir M, Thebo KH et al (2020) Laminar graphene oxide membranes towards selective ionic and molecular separations: challenges and progress. Chem Rec 20(4):344–354
Hossain S, Abdalla AM, Suhaili SB et al (2020) Nanostructured graphene materials utilization in fuel cells and batteries: a review. J Energy Storage 29:101386
Park C, Lee E, Lee G et al (2020) Superior durability and stability of Pt electrocatalyst on N-doped graphene-TiO2 hybrid material for oxygen reduction reaction and polymer electrolyte membrane fuel cells. Appl Catal B Environ 268:118414
Lavanya G, Paradesi D, Hemalatha P (2020) Development of proton conducting polymer nanocomposite membranes based on SPVdF-HFP and graphene oxide for H2-O2 fuel cells. J Macromol Sci A 57(4):283–290
Poolnapol L, Kao-ian W, Somwangthanaroj A et al (2020) Silver decorated reduced graphene oxide as electrocatalyst for zinc–air batteries. Energies 13(2):462
ElMekawy A, Hegab HM, Losic D et al (2017) Applications of graphene in microbial fuel cells: the gap between promise and reality. Renew Sust Energ Rev 72:1389–1403
Bollella P, Fusco G, Tortolini C et al (2017) Beyond graphene: electrochemical sensors and biosensors for biomarkers detection. J Biosens Bioelectron 89(1):152–166
Call TP, Carey T, Bombelli P et al (2017) Platinum-free, graphene based anodes and air cathodes for single chamber microbial fuel cells. J Mater Chem A 5(45):23872–23886
Choi HJ, Jung SM, Seo JM et al (2012) Graphene for energy conversion and storage in fuel cells and supercapacitors. Nano Energy 1(4):534–551
Lyu H (2020) Triple layer tungsten trioxide, graphene, and polyaniline composite films for combined energy storage and electrochromic applications. Polymers 12(1):49
Wang B, Ruan T, Chen Y et al (2020) Graphene-based composites for electrochemical energy storage. Energy Storage Mater 24:22–51
Dutta D, Jiang JY, Jamaluddin A et al (2019) Nanocatalyst-assisted fine tailoring of pore structure in holey-graphene for enhanced performance in energy storage. ACS appl mater inters 11(40):36560–36570
Qamar S, Akhtar MN, Aleem W et al (2020) Graphene anchored Ce doped spinel ferrites for practical and technological applications. Ceram Int 46(6):7081–7088
Habib MM, Roy R, Islam MM et al (2019) Study of graphene-MoS2 based SPR biosensor with graphene based SPR biosensor: comparative approach. Int J Nat Sci Res 7(1):1–9
de Freitas ME, Amorim RG, Feliciano GT et al (2020) The role of water on the electronic transport in graphene nanogap devices designed for DNA sequencing. Carbon 158:314–319
Wasfi A, Awwad F, Ayesh AI (2020) DNA sequencing via Z-shaped graphene nanoribbon field effect transistor decorated with nanoparticles using first-principle transport simulations. New J Phys 22(6):063004
Bernardo P, Drioli E, Golemme G (2009) Membrane gas separation: a review/state of the art. J Ind Eng Chem Res 48(10):4638–4663
Novoselov KS, Geim AK, Morozov SV et al (2004) Electric field effect in atomically thin carbon films. Science 306(5696):666–669
Oyama ST, Lee D, Hacarlioglu P et al (2004) Theory of hydrogen permeability in nanoporous silica membranes. J Membr Sci 244(1–2):244–245
Jiang DE, Cooper VR, Dai S (2009) Porous graphene as the ultimate membrane for gas separation. Nano Lett 9(12):4019–4024
Du H, Li J, Zhang J et al (2011) Separation of hydrogen and nitrogen gases with porous graphene membrane. J Phys Chem C 115(47):23261–23266
Hauser AW, Schwerdtfeger P (2012) Methane-selective nanoporous graphene membranes for gas purification. Phys Chem Chem Phys 14(38):13292–13298
Hou Y, Li Y, Jiang C et al (2019) Molecular simulation for separation of ethylene and ethane by functionalised graphene membrane. Mol Simul 45(16):1322–1331
Wu T, Xue Q, Ling C et al (2014) Fluorine-modified porous graphene as membrane for CO2/N2 separation: molecular dynamic and first-principles simulations. J Phys Chem C 118(14):7369–7376
Lei G, Liu C, Xie H et al (2014) Separation of the hydrogen sulfide and methane mixture by the porous graphene membrane: effect of the charges. Chem Phys Lett 599:127–132
Hauser AW, Mitrushchenkov AO, de Lara-Castells MP (2017) Quantum nuclear motion of helium and molecular nitrogen clusters in carbon nanotubes. J Phys Chem C 121(7):3807–3821
Schrier J (2010) Helium separation using porous graphene membranes. J Phys Chem Lett 1(15):2284–2287
Alnssar KN, Roknabadi MR, Behdani M et al (2020) Theoretical study of electronic properties of nanostructures composed of blue phosphorene and graphene sheet. In IOP conference series: Mater Sci Eng 871(1): p.012084. IOP Publishing
Jadoon T, Carter-Fenk K, Siddique MB et al (2020) Silver clusters tune up electronic properties of graphene nanoflakes: a comprehensive theoretical study. J Mol Liq 297:111902
Rossi-Fernández AC, Villegas-Escobar N, Guzmán-Angel D et al (2020) Theoretical study of glycine amino acid adsorption on graphene oxide. J Mol Model 26(2):33
Elgengehi SM, El-Taher S, Ibrahim MA et al (2020) Graphene and graphene oxide as adsorbents for cadmium and lead heavy metals: a theoretical investigation. Appl Surf Sci 507:145038
Koçak İ, Alıcı H (2020) Experimental and theoretical studies of electrochemical oxidation of nicotinamide adenine dinucleotide at the modified SWCNT and graphene oxide. J Mol Model 26(3):51
Wei S, Zhou S, Wu Z, Wang M et al (2018) Mechanistic insights into porous graphene membranes for helium separation and hydrogen purification. Appl Surf Sci 441:631–638
Burke K (2012) Perspective on density functional theory. J Chem Phys 136(15):150901
Ambrosetti A, Silvestrelli PL (2014) Gas separation in nanoporous graphene from first principle calculations. J Phys Chem C 118(33):19172–19179
Lu R, Rao D, Lu Z et al (2012) Prominently improved hydrogen purification and dispersive metal binding for hydrogen storage by substitutional doping in porous graphene. J Phys Chem C 116(40):21291–21296
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti Correlation-Energy Formula into a functional of the electron density. Phys Rev B 37(2):785–789
Dunning Jr TH (1989) Gaussian basis sets for use in correlated molecular calculations. I The Atoms Boron Through Neon and Hydrogen J Chem Phys 90(2):1007–1023
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27(15):1787–1799
Ehrlich S, Bollmann J, Reckien W, Bredow T, Grimme S (2011) System-dependent dispersion coefficients for the DFT-D3 treatment of adsorption processes on ionic surfaces. Chem Phys Chem 12(17):3414–3420
Leenaerts O, Partoens B, Peeters F (2009) Adsorption of small molecules on graphene. Microelectron J 40(4–5):860–862
Ortiz YP, Jalbout AF (2013) Graphene metal adsorption as a model chemistry for atmospheric reactions. Chem Phys Lett 564:73–77
Chen L, Xu C, Zhang XF et al (2009) Raman and infrared-active modes in MgO nanotubes. Physica E Low dimens Syst Nanostruct 41(5):852–855
Beheshtian J, Kamfiroozi M, Bagheri Z et al (2012) Theoretical study of hydrogen adsorption on the B12P12 fullerene-like nanocluster. Comput Mater Sci 54:115–118
Trani F, Causà M, Lettieri S et al (2009) Role of surface oxygen vacancies in photoluminescence of tin dioxide nanobelts. Maddalena, Microelectron J Comput Mater Sci 40(2):236–238
Dinadayalane TC, Murray JS, Concha MC et al (2010) Reactivities of sites on (5, 5) single-walled carbon nanotubes with and without a Stone-Wales defect. J Chem Theory Comput 6(4):1351–1357
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian 98, Rev A.7. Gaussian Inc, Pittsburgh PA
Shahbabaei M, Tang D, Kim D (2017) Simulation insight into water transport mechanisms through multilayer graphene-based membrane. J Comput Mater Sci 128:87–97
Shan M, Xue Q, Jing N et al (2012) Influence of chemical functionalization on the CO2/N2 separation performance of porous graphene membranes. Nanoscale 4(17):5477–5482
Hauser AW, Schrier J, Schwerdtfeger P (2012) Helium tunneling through nitrogen-functionalized graphene pores: pressure- and temperature-driven approaches to isotope separation. J Phys Chem C 116(19):10819–10827
Hobza P, Zahradnik R (1988) Intermolecular interactions between medium-sized systems. Nonempirical and empirical calculations of interaction energies. Successes and failures J Chem Rev 88(6):871–897
Navarrete M, Rangel C, Corchado JC et al (2005) Trapping of the OH radical by α-tocopherol: a theoretical study. J Phys Chem A 109(21):4777–4784
Zhang HY, Ji HF (2003) S-H proton dissociation enthalpies of thiophenolic cation radicals: a DFT study. J Mol Struct : THEOCHEM 663(1–3):167–174
Chandra AK, Uchimaru T (2002) The OH bond dissociation energies of substituted phenols and proton affinities of substituted phenoxide ions: a DFT study. Int J Mol Sci 3(4):407–422
Code availability
The calculations have been carried out using the Chemistry Computation Center at Shahid Beheshti University by employing Gaussian 003 and Gauss View Version 6 provided by Gaussian, Inc.
Funding
This research was funded by the Research Council of Alzahra University (Grant No. 1431).
Author information
Authors and Affiliations
Contributions
Mina Ghiasi: Conceptualization, Methodology, Software, Writing- Reviewing and Editing, Supervision, Validation.
Parisa Zeinali: Data curation, Writing- Original draft, Software.
Samira Gholami: Data curation, Writing- Original draft, Software.
Mansour Zahedi: Software, Reviewing and Editing
Corresponding author
Ethics declarations
Ethics approval
The manuscript is prepared in compliance with the Ethics in Publishing Policy as described in the Guide for Authors.
Consent to participate
The manuscript is approved by all authors for publication.
Consent for publication
The consent for publication was obtained from all participants.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghiasi, M., Zeinali, P., Gholami, S. et al. Separation of CH4, H2S, N2 and CO2 gases using four types of nanoporous graphene cluster model: a quantum chemical investigation. J Mol Model 27, 201 (2021). https://doi.org/10.1007/s00894-021-04812-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04812-2