Abstract
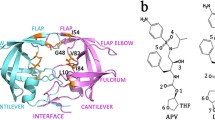
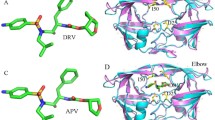
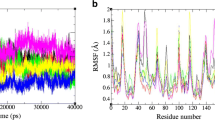
Residue Gly86 is considered as the highly conversed residue in the HIV-1 protease. In our work, the detailed binding free energies for the wild-type (WT) and mutated proteases binding to the TMC-114 are estimated to investigate the protein-inhibitor binding and drug resistance mechanism by molecule dynamic simulations and molecular mechanics Poisson Boltzmann surface area (MM-PBSA) method. The binding affinities between the mutants and inhibitor are different than that in the wild-type complex and the major resistance to Darunavir (DRV) of G86A and G86S originate from the electrostatic energy and entropy, respectively. Furthermore, free energy decomposition analysis for the WT and mutated complexes on the basis of per-residue indicates that the mutagenesis influences the energy contribution of the residue located at three regions: active site region (residue 24–32), the flap region, and the region around the mutated residue G86 (residue 79–88), especially the flap region. Finally, further hydrogen bonds and structure analysis are carried out to detect the relationship between the energy and conformation. In all, the G86 mutations change the flap region’s conformation. The experimental results are in good agreement with available results.






Similar content being viewed by others
References
Wlodawer A (2002) Rational approach to AIDS drug design through structural biology. Annu Rev Med 53:595–614
Swain AL, Miller MM, Green J, Rich DH, Schneider J, Kent SBH, Wlodawer A (1990) X-ray crystallographic structure of a complex between a synthetic protease of human immunodeficiency virus-1 and a substrate-based hydroxyethylamine inhibitor. Proc Natl Acad Sci U S A 87:8805–8809
Wlodawer A, Miller M, Jaskolski M, Sathyanarayana BK, Baldwin E, Weber IT, Selk LM, Clawson L, Schneider J, Kent SBH (1989) Conserved folding in retroviral proteases-crystal-structure of a synthetic HIV-1 protease. Science 245:616–621
Navia MA, Fitzgerald PMD, Mckeever BM, Leu CT, Heimbach JC, Herber WK, Sigal IS, Darke PL, Springer JP (1989) 3-dimensional structure of aspartyl protease from human immunodeficiency virus Hiv-1. Nature 337:615–620
Barbaro G, Scozzafava A, Mastrolorenzo A, Supuran CT (2005) Highly active antiretroviral therapy: current state of the art, new agents and their pharmacological interactions useful for improving therapeutic outcome. Curr Pharm Des 11:1805–1843
Surleraux DLNG, Tahri A, Verschueren WG, Pille GME, Kock HA, Jonckers THM, Peeters A, Meyer DS, Azijn H, Pauwels R, de Bethune MP, King NM, Prabu-Jeyabalan M, Schiffer CA, Wigerinck PBTP (2005) Discovery and selection of TMC114, a next generation HIV-1 protease inhibitor. J Med Chem 48:1813–1822
Chen RX, Quinones-Mateu ME, Mansky LM (2004) Drug resistance, virus fitness and HIV-1 mutagenesis. Curr Pharm Des 10:4065–4070
Clavel F, Hance AJ (2004) Medical progress: HIV drug resistance. N Engl J Med 350:1023–1035
Ishima R, Gong Q et al (2010) Highly conserved glycine 86 and arginine 87 residues contribute differently to the structure and activity of the mature HIV-1 protease. Proteins 78:1015–1025
Hou TJ, Yu R (2007) Molecular dynamics and free energy studies on the wild-type and double mutant HIV-1 protease complexed with amprenavir and two amprenavir-related inhibitors: mechanism for binding and drug resistance. J Med Chem 50:1177–1188
Meher BR, Wang Y (2012) Interaction of I50V mutant and I50L/A71V double mutant HIV-protease with inhibitor TMC114 (Darunavir): molecular dynamics simulation and binding free energy studies. J Phys Chem B 116:1884–1900
Pearl LH, Taylor WR (1987) A structural model for the retroviral proteases. Nature 329:351–354
Rao JKM, Erickson JW, Wlodawer A (1991) Structural and evolutionary relationships between retroviral an eucaryotic aspartic proteinases. Biochemistry 30:4663–4671
Loeb DD, Swanstrom R, Everitt L, Manchester M, Stamper SE, Hutchison CA III (1989) Complete mutagenesis of the HIV-1 protease. Nature 340:397–400
Shao W, Everitt L, Manchester M, Loeb DD, Hutchison CA III, Swanstrom R (1997) Sequence requirements of the HIV-1 protease flap region determined by saturation mutagenesis and kinetic analysis of flap mutants. Proc Natl Acad Sci U S A 94:2243–2248
Weber IT (1990) Comparison of the crystal structures and intersubunit interactions of human immunodeficiency and Rous sarcoma virus proteases. J Biol Chem 265:10492–10496
Ishima R, Torchia DA, Louis JM (2007) Mutational and structural studies aimed at characterizing the monomer of HIV-1 protease and its precursor. J Biol Chem 282:17190–17199
Louis JM, Ishima R, Nesheiwat I, Pannell LK, Lynch SM, Torchia DA, Gronenborn AM (2003) Revisiting monomeric HIV-1 protease. Characterization and redesign for improved properties. J Biol Chem 278:6085–6092
Mahalingam B, Louis JM, Hung J, Harrison RW, Weber IT (2001) Structural implications of drug-resistant mutants of HIV-1 protease: high-resolution crystal structures of the mutant protease/substrate analogue complexes. Proteins 43:455–464
Adcock SA, McCammon JA (2006) Molecular dynamics: survey of methods for simulating the activity of proteins. Chem Rev 106:1589–1615
Kollman PA, Massova I, Reyes C, Kuhn B, Huo SH, Chong L, Lee M, Lee T, Duan Y, Wang W, Donini O, Cieplak P, Srinivasan J, Case DA, Cheatham TE (2000) Calculating structures and free energies of complex molecules: combining molecular mechanics and continuum models. Acc Chem Res 33:889–897
Li L, Chen H, Zhao RN, Han JG (2013) The investigations on HIV-1 gp120 bound with BMS-488043 by using docking and molecular dynamics simulations. J Mol Model 19:905–917
Wang W, Kollman PA (2000) Free energy calculations on dimer stability of the HIV protease using molecular dynamics and a continuum solvent model. J Mol Biol 303:567–582
Wang W, Kollman PA (2001) Computational study of protein specificity: the molecular basis of HIV-1 protease drug resistance. Proc Natl Acad Sci U S A 98:14937–14942
Li D, Han JG, Chen H, Li L, Zhao RN, Liu G, Duan Y (2012) Insights into the structural function of the complex of HIV-1 protease with TMC-126: molecular dynamics simulations and free-energy calculations. J Mol Model 18:1841–1845
Wang J, Morin P, Wang W, Kollman PA (2001) Use of MM-PBSA in reproducing the binding free energies to HIV-1RT of TIBO derivates and predicting the binding mode to HIV-1 RTof Efavirenz by docking and MM-PBSA. J Am Chem Soc 123:5221–5230
Kuhn B, Gerber P, Schulz-Gasch T, Stahl M (2005) Validation and use of the MM-PBSA approach for drug discovery. J Med Chem 48:4040–4048
Huo S, Wang J, Cieplak P, Kollman PA, Kuntz ID (2002) Molecular dynamics and free energy analyses of cathepsin D-inhibitor interactions: insight into structure-based ligand design. J Med Chem 45:1412–1419
Kollman PA (1993) Free-energy calculations-applications to chemical and biochemical phenomena. Chem Rev 93:2395–2417
Gohlke H, Kiel C, Case DA (2003) Insights into protein–protein binding by binding free energy calculation and free energy decomposition for the Ras-Raf and Ras-RalGDS complexes. J Mol Biol 330:891–913
Zoete V, Meuwly M, Karplus M (2005) Study of the insulin dimerization: binding free energy calculations and per-residue free energy decomposition. Proteins 61:79–93
Chen XN, Tropsha A (1995) Relative binding free energies of peptide inhibitors of HIV-1 protease: the influence of the active site protonation state. J Med Chem 38:42–48
Tie Y, Boross PI, Wang YF, Gaddis L, Hussain AK, Leshchenko S, Ghosh AK, Louis JM, Harrison RW, Weber IT (2004) High resolution crystal structures of HIV-1 protease with a potent non-peptide inhibitor [UIC-94017] active against multidrug-resistant clinical strains. J Mol Biol 338:341–352
Kovalevsky AY, Tie Y, Liu F, Boross PI, Wang YF, Leshchenko S, Ghosh AK, Harrison RW, Weber IT (2006) Effectiveness of nonpeptide clinical inhibitor TMC-114 on HIV-1 protease with highly drug resistant mutations D30N, I50 V, and L90M. J Med Chem 49:1379–1387
Case DA, Darden TA, Cheatham TE III, Simmerling CL, Wang J, Duke RE, Luo R, Crowley M, Walker RC, Zhang W, Merz KM, Wang B, Hayik S, Roitberg A, Seabra G, Kolossváry I, Wong KF, Paesani F, Vanicek J, Wu X, Brozell SR, Steinbrecher T, Gohlke H, Yang L, Tan C, Mongan J, Hornak V, Cui G, Mathews DH, Seetin MG, Sagui C, Babin V, Kollman PA (2008) AMBER 10. University of California, San Francisco
Wang JM, Wolf RM, Caldwell JW, Kollman PA, Case DA (2004) Development and testing of a general amber force field. J Comput Chem 25:1157–1174
Jakalian A, Bush BL, Jack DB, Bayly CI (2000) Fast, efficient generation of high-quality atomic charges. AM1-BCC model: I. Method. J Comput Chem 21:132–146
Duan Y, Wu C, Chowdhury S, Lee MC, Xiong GM, Zhang W, Yang R, Cieplak P, Luo R, Lee T, Caldwell J, Wang JM, Kollman P (2003) A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J Comput Chem 24:1999–2012
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935
Darden T, York D, Pedersen L (1993) Particle Mesh Ewald–an N. Log-[N] method for Ewald sums in large systems. J Chem Phys 98:10089–10092
Ryckaert JP, Ciccotti G, Berendsen HJC (1977) Numerical-integration of Cartesian equations of motion of a system with constraints-molecular-dynamics of N-alkanes. J Comput Phys 23:327–341
Weiser J, Shenkin PS, Still WC (1999) Approximate atomic surfaces from linear combinations of pairwise overlaps (LCPO). J Comput Chem 20:217–230
Onufriev A, Bashford D, Case DA (2000) Modification of the generalized born model suitable for macromolecules. J Phys Chem B 104:3712–3720
Case DA, Cheatham TE, Darden T, Gohlke H, Luo R, Merz KM, Onufriev A, Simmerling C, Wang B, Woods RJ (2005) The Amber biomolecular simulation programs. J Comput Chem 26:1668–1688
Freedberg DI, Wang YX, Stahl SJ, Kaufman JD, Wingfield PT, Kiso Y, Torchia DA (1998) Flexibility and function in HIV protease: dynamics of the HIV-1 protease bound to the asymmetric inhibitor kynostatin 272 [KNI-272]. J Am Chem Soc 120:7916–7923
Zoete V, Michielin O, Karplus M (2002) Relation between sequence and structure of HIV-1 protease inhibitor complexes: a model system for the analysis of protein flexibility. J Mol Biol 315:21–52
Acknowledgments
This work is supported by Natural Scientific Fund of China (11179035) and 973 fund of Chinese Ministry of Science and Technology (2010CB934504). This paper has been performed partly on the Supercomputing Center of University of Science and Technology of China.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, D., Zhang, Y., Zhao, RN. et al. Investigation on the mechanism for the binding and drug resistance of wild type and mutations of G86 residue in HIV-1 protease complexed with Darunavir by molecular dynamic simulation and free energy calculation. J Mol Model 20, 2122 (2014). https://doi.org/10.1007/s00894-014-2122-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2122-y