Abstract
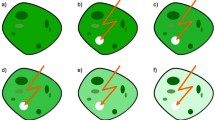
Fluorescence loss in photobleaching (FLIP) is a modern microscopy method for visualization of transport processes in living cells. Although FLIP is widespread, an automated reliable analysis of image data is still lacking. This paper presents a framework for modeling and simulation of FLIP sequences as reaction–diffusion systems on segmented cell images. The cell geometry is extracted from microscopy images using the Chan–Vese active contours algorithm (IEEE Trans Image Process 10(2):266–277, 2001). The PDE model is subsequently solved by the automated Finite Element software package FEniCS (Logg et al. in Automated solution of differential equations by the finite element method. Springer, Heidelberg, 2012). The flexibility of FEniCS allows for spatially resolved reaction diffusion coefficients in two (or more) spatial dimensions.


























Similar content being viewed by others
References
Aridor, M., Hannan, L.A.: Traffic Jams II: an update of diseases of intracellular transport. Traffic 3(11), 781–790 (2002)
Bancaud, A., Huet, S., Daigle, N., Mozziconacci, J., Beaudouin, J., Ellenberg, J.: Molecular crowding affects diffusion and binding of nuclear proteins in heterochromatin and reveals the fractal organization of chromatin. EMBO J. 28, 3785–3798 (2009)
Beaudouin, J., Mora-Bermúdez, F., Klee, T., Daigle, N., Ellenberg, J.: Dissecting the contribution of diffusion and interactions to the mobility of nuclear proteins. Biophys. J. 90, 1878–1894 (2006)
Bohl, E.: Finite Modelle gewönlicher Randwertaufgaben. Teubner, Stuttgart (1981)
Bohl E.: Monotonie: Lösbarkeit und Numerik bei Operatorgleichungen, Springer Tracts in Natural Philosophy 25, Berlin (1974)
Braga, J., Desterro, J.M., Carmo-Fonseca, M.: Intracellular macromolecular mobility measured by fluorescence recovery after photobleaching with confocal laser scanning microscopes. Mol. Biol. Cell 15, 4749–4760 (2004)
Chan, T., Vese, L.: Active contours without edges. IEEE Trans. Image Process. 10(2), 266–277 (2001)
Collatz, L.: Funktionalanalysis und Numerische Mathematik. Springer, Berlin (1964)
Davis, T.A.: Algorithm 832. ACM Trans. Math. Softw. 30(2), 196–199 (2004)
Geuzaine, Ch., Remacle, J.F.: Gmsh. http://geuz.org/gmsh
Ghosh, S.K., Cherstvy, A.G., Metzler, R.: Non-universal tracer diffusion in crowded media of non-inert obstacles. Phys. Chem. Chem. Phys. 17, 1847–1858 (2015)
Hairer, E., Wanner, G.: Solving Ordinary Differential Equations II. Springer, Heidelberg (1996)
Hansen, ChV: Segmentation of Fluorescent Microscopy Images of Living Cells. Bachelor Project, IMADA, SDU (2012)
Hinde, E., Cardarelli, F., Digman, M.A., Gratton, E.: In vivo pair correlation analysis of EGFP intranuclear diffusion reveals DNA-dependent molecular flow. Proc. Natl. Acad. Sci. USA 107, 16560–16565 (2010)
Istratov, A.A., Vyvenko, O.F.: Exponential analysis in physical phenomena. Rev. Sci. Instr. 70, 1233–1257 (1999)
Jönsson, P., Jonsson, M.P., Tegenfeldt, J.O., Hk, F.: A method improving the accuracy of fluorescence recovery after photobleaching analysis. Biophys. J. 95, 5334–5348 (2008)
Kass, M., Witkin, A., Terzopoulos, D.: Snakes: active contour models. Int. J. Comput. Vis. 1, 321–331 (1988)
Lippincott-Schwartz, J., Altan-Bonnet, N., Patterson, G.H.: Photobleaching and photoactivation: following protein dynamics in living cells. Nat. Cell Biol. Suppl. 7, S7–14 (2003)
Logg, A., Mardal, K.-A., Wells, G.N., et al.: Automated Solution of Differential Equations by the Finite Element Method. Springer, Heidelberg (2012)
Lund, F.W., Wüstner, D.: A comparison of single particle tracking and temporal image correlation spectroscopy for quantitative analysis of endosome motility. J. Microsc. 252, 169–188 (2013)
McWhirter, J.G., Pike, E.R.: On the numerical inversion of the Laplace transform and similar Fredholm integral equations of the first kind. J. Phys. A Math. Gen. 11, 1729–1745 (1978)
Müller, F., Mazza, D., Stasevich, T.J., McNally, J.G.: FRAP and kinetic modeling in the analysis of nuclear protein dynamics: what do we really know? Curr. Opin. Cell Biol. 22, 403–411 (2010)
Portable, Extensible Toolkit for Scientific Computation: url = http://www.mcs.anl.gov/petsc
Sprague, B.L., Pego, R.L., Stavreva, D.A., McNally, J.G.: Analysis of binding reactions by fluorescence recovery after photobleaching. Biophys. J. 86, 3473–3495 (2004)
Stasevich, T.J., Mller, F., Brown, D.T., McNally, J.G.: Dissecting the binding mechanism of the linker histone in live cells: an integrated FRAP analysis. EMBO J. 29, 1225–1234 (2010)
Varga, R.S.: Matrix Iterative Analysis. Prentice-Hall, Englewood Cliffs (1962)
Vo, G.D.: Comparison of continuous and discontinuous Galerkin finite element methods for parabolic partial differential equations with implicit time stepping. M.S. thesis, Montana State University (2012)
Wüstner, D., Solanko, L.M., Lund, F.W., Sage, D., Schroll, H.J., Lomholt, M.A.: Quantitative fluorescence loss in photobleaching for analysis of protein transport and aggregation. BMC Bioinform. 13, 296 (2012)
Zilman, A., Talia, S.D., Chait, B.T., Rout, M.P., Magnasco, M.O.: Efficiency, selectivity, and robustness of nucleocytoplasmic transport. PLoS Comp. Biol. 3(7), e125 (2007)
Acknowledgments
The authors want to thank Niels Christian Overgaard from Lund University for introducing us to level set methods in image segmentation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Gabriel Wittum.
Appendices
Appendix 1: Shape gradients
The Chan–Vese energy (2) consists of two parts: Minimizing the length of the curve has some regularizing effect \(E_{\mathrm {reg}}(\varGamma ) = \int _\varGamma \; \mathrm {d} \sigma \). Without loss of generality, we may assume parametrization with respect to arc-length
such that \(|\gamma '|=1\) and \(l = \int _\varGamma \; \mathrm {d} \sigma = \int _0^l |\gamma '(\sigma )|^2 \; \mathrm {d} \sigma \). Let \(\omega \) be another closed, \(C^1\)-curve \(\omega : [0,l] \rightarrow {\mathbb R}^2\) such that \(\omega (0)=\omega (l)\) and \(\omega '(0)=\omega '(l)\). Consider an \(\epsilon \)-variation of the curve \(\varGamma \) given by \(\gamma _\epsilon = \gamma + \epsilon \omega \). The variation of \(E_{reg} = \int _0^l |\gamma '(\sigma )|^2 \; \mathrm {d} \sigma \) is defined as
By differentiation we find
By partial integration, using periodicity of \(\gamma '\) and \(\omega \)
By definition, the \(L^2\)-shape gradient of \(E_{\mathrm {reg}}\) is
In arc-length parametrization \(\gamma '' = - \kappa \cdot \mathbf {n}\), where \(\mathbf {n}\) is the outward unit normal. Finally
The fitting energy is
The integral over the exterior segment equals the integral over the entire image without the interior part
The last integral does not depend on the curve \(\varGamma \) and the first one is subject of the next Lemma.
Lemma 1
Let \(\gamma \) and \(\omega \) be closed \(C^1\)-curves. Let \(V : {\mathbb R}^2 \rightarrow {\mathbb R}\) be integrable. The variation of
is
The proof below shows that for Lemma 1 to hold true, arc-length parametrization is not necessary but convenient. Otherwise the unit normal n is to be replaced by the non-unit outward normal. As a result we have that the \(L^2\)-shape gradient is given by
Proof of Lemma 1
Let \(P(x_1,x_2)=0\) and \(Q(x_1,x_2)=\int _{0}^{x_1} V(\xi ,x_2) \; {\mathrm {d}} \xi \).
By Green’s formula
In the last curve integral \(\mathbf {x}=\gamma \), \({\mathrm {d}} x_2 = \gamma _2' \; \mathrm {d} \sigma \) and thus
Consider the \(\epsilon \)-variation \(\gamma _\epsilon = \gamma + \epsilon \omega \) and find
By partial integration and periodicity
Here the (unit) normal is given by \(\mathbf {n}= (\gamma _2', -\gamma _1')^T\) and our proof is complete. \(\square \)
The shape gradient of the Chan–Vese energy (3) follows from (23) and (24) where \(V(\mathbf {x}) = (I(\mathbf {x})-c_-)^2 - (I(\mathbf {x})-c_+)^2\).
Appendix 2: Stability analysis
For simplicity of presentation we consider a reaction–diffusion system of type (9), but in one spatial variable and with diffusion coefficients scaled to unity
For illustration we consider homogenous Dirichlet and Neumann conditions for u and v, respectively
The interested reader can easily replace the Dirichlet condition by a Neumann condition as in (11). Initial data is given as in (12). The analysis uses elements by E. Bohl [4] which originally go back to R.S. Varga [26], L. Collatz [8] and E. Bohl [5]. We have chosen to present this concept, because it generalizes to two dimensions and applies to non-linear reaction diffusion problems.
The bilinear form
acting on a finite dimensional approximation \(u(x) = \sum _j u_j \phi _j(x)\) with piecewise linear elements \(\phi _j(x_i) = \delta _{ij}\) is expressed as matrix–vector product
The vector \(\mathbf {u}\) holds approximations on inner grid points \(\mathbf {u}= (u_i)\), \(u_i = u(x_i)\), \(x_i=ih\), \(i=1,2,\ldots , m\), and \((m+1)h=1\). The matrix is given by
Due to the piecewise constant gradients \(\nabla \phi _j\), the matrix becomes a well-known (finite difference) approximation to the second derivative. With homogenous Dirichlet conditions the linear operator reads
In the case of homogenous Neumann conditions \(v_0=v_1\) and \(v_m=v_{m+1}\), the second derivative on inner grid points is approximated by
Semi-implicit time stepping results in a linearly coupled, discrete system
Naturally, step sizes are positive \(\varDelta {t}\), \(h > 0\) and reaction rates are non-negative \(k^\pm \ge 0\). Again, \(u_i^n\) is thought as approximation to \(u(t_n,x_i)\). In fact one can prove that the numerical approximation converges to the solution of the PDE system.
The key argument relies on the fact that the finite difference operators \(\mathbf {Id}+ \varDelta {t}\mathbf {A}_h\) as well as \(\mathbf {Id}+ \varDelta {t}\mathbf {B}_h\) are inverse-monotone with bounded inverse. While \(\mathbf {A}_h\) inherits its inverse-monotonicity from the negative Laplacian, \(\mathbf {B}_h\) is obviously singular.
Lemma 2
With non-negative parameters \(\varDelta {t}\ge 0\), \(h>0\) and reaction rates \(k^\pm \ge 0\) both system matrices \((1+\varDelta {t}k^+)\mathbf {Id}+ \varDelta {t}\mathbf {A}_h\) and \((1+\varDelta {t}k^-)\mathbf {Id}+ \varDelta {t}\mathbf {B}_h\) are regular with non-negative and uniformly bounded inverse.
An obvious consequence is:
Corollary 1
With non-negative initial data \(u^0 \ge 0\) and \(v^0 \ge 0\) and reaction rates \(k^\pm \ge 0\), approximations computed by (26) remain non-negative for all discrete time levels \(n \varDelta {t}\ge 0\).
The proof of Lemma 2 is based on inverse-monotone Z-matrices. A Z-matrix is characterized by its non-positive off-diagonal elements:
A matrix \(\mathbf {A}\) is called inverse-monotone if it is invertible with elementwise non-negative inverse \(\mathbf {A}^{-1} \ge 0\). In this case \(\mathbf {A}\mathbf {e}\ge 0\) implies \(\mathbf {e}\ge 0\); i.e. solving the system preserves positivity. An inverse-monotone Z-matrix is called M-matrix.
Any strictly positive vector \(\mathbf {p}>0\) defines a weighted max-norm
The corresponding operator norm for a monotone (i.e. elementwise positive) matrix is \(\Vert \mathbf {A}\Vert _p = \Vert \mathbf {A}\mathbf {p}\Vert _p\).
The following M-criterion can be found in E. Bohl [4], Chapter I, Theorem 5.1:
Lemma 3
A Z-matrix \(\mathbf {A}\) is M-matrix if and only if \(\mathbf {A}\) is semi-positive. That is, there exists \(\mathbf {e}>0\) with \(\mathbf {A}\mathbf {e}> 0\).
Obviously, adding a non-negative diagonal matrix to a M-matrix preserves the M-property. With these preparations, we are ready to prove the main stability result.
Proof of Lemma 2
Let \(\delta = (1,\ldots ,1)^T\). Note that \(\mathbf {A}_h\delta = (1,0,\ldots ,0,1)/h^2\) and \(B_h\delta = 0\). Obviously \(\mathbf {B}_h\) is singular. Both \(\mathbf {Id}+\varDelta {t}\mathbf {A}_h\) and \(\mathbf {Id}+\varDelta {t}\mathbf {B}_h\) are Z-matrices. Both are semi-positive
By Lemma 3 \(\mathbf {Id}+\varDelta {t}\mathbf {A}_h\) and \(\mathbf {Id}+\varDelta {t}\mathbf {B}_h\) are M-matrices. We may add any positive diagonal matrix \(\varDelta {t}k \mathbf {Id}\) and the M-property is preserved. Thus both system matrices are M-matrices, even with non-constant, non-negative reaction rates \(k^\pm \). It remains to show the uniform bound for the inverse. We have that
Multiplying by the non-negative inverse, taking norms the desired bound follows
and the proof is complete. \(\square \)
Note that \(\Vert \cdot \Vert _\delta \) is the well-known max-norm. Via the eigensystem of \(\mathbf {A}_h\) one can also show that \(\mathbf {A}_h\) itself is a M-matrix.
The uniform bound of the inverse system matrix implies stability of the numerical method: By linearity, errors are governed by the system (26) itself. Uniform boundedness (27) implies stability in the sense that errors at later time depend continuously on initial errors. Convergence towards a smooth solution of the reaction–diffusion system (25) follows from the discrete Gronwall lemma.
Rights and permissions
About this article
Cite this article
Hansen, C.V., Schroll, H.J. & Wüstner, D. Computational modeling of fluorescence loss in photobleaching. Comput. Visual Sci. 17, 151–166 (2015). https://doi.org/10.1007/s00791-015-0259-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00791-015-0259-6