Abstract
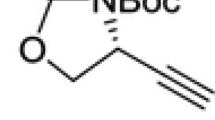
The ethynylglycine synthon {(R)-2,2-dimethyl-3-(tert-butoxycarbonyl)-4-ethynyl-oxazolidine} is a chiral compound with valuable synthetic interest. An update (covering literature from 2005 to 2017) on the different synthetic utilities is reviewed and discussed.




































Similar content being viewed by others
Abbreviations
- Ac:
-
Acetyl
- ACC synthase:
-
1-Aminocyclopropane-1-carboxylate synthase
- All:
-
Allyl
- Bn:
-
Benzyl
- Boc:
-
tert-Butoxycarbonyl
- Bu or n-Bu:
-
n-Butyl
- CAN:
-
Cerium ammonium nitrate
- Cbz:
-
Benzyloxycarbonyl
- dba:
-
Dibenzylideneacetone
- DCC:
-
N,N′-Dicyclohexylcarbodiimide
- DIBAL-H:
-
Diisobutylalumino hydride
- DIPA:
-
Diisopropylamine
- DIPEA:
-
Diisopropylethylamine
- DMAP:
-
4-Dimethylaminopyridine
- DMF:
-
Dimethylformamide
- DMP:
-
Dess–Martin periodinane
- ent-x :
-
Enantiomer of compound x
- Et:
-
Ethyl
- HMDS:
-
Hexamethyldisilazane
- IDO:
-
Indoleamine 2,3-dioxygenase
- LHMDS:
-
Lithium bis(trimethylsilyl)amide
- MCPBA:
-
m-Chloroperoxybenzoic acid
- Me:
-
Methyl
- Mts:
-
2,4,6-Trimethylbenzenesulfonyl
- MW:
-
Microwave
- NCS:
-
N-Chlorosuccinimide
- oDPPBA:
-
2-(Diphenylphosphino)benzoic acid
- oDPPB:
-
2-(Diphenylphosphino)benzoate
- Ph:
-
Phenyl
- PLP:
-
Pyridoxal phosphate
- PTSA:
-
p-Toluenesulfonic acid
- RCM:
-
Ring-closing metathesis
- SEM:
-
2-(Trimethylsilyl)ethoxymethyl
- TBAF:
-
Tetrabutylammonium fluoride
- TBAI:
-
Tetrabutylammonium iodide
- TBDMS:
-
tert-butyldimethylsilyl
- TBDPS:
-
tert-butyldiphenylsilyl
- t-Bu:
-
tert-butyl
- TEMPO:
-
2,2,6,6-Tetramethylpiperidine 1-oxyl
- TES:
-
Triethylsilyl
- Tf:
-
Triflate
- TFA:
-
Trifluoroacetic acid
- THF:
-
Tetrahydrofuran
- TMEDA:
-
N,N,N′,N′-tetramethyl ethylenediamine
- TMS:
-
Trimethylsilyl
- Ts:
-
4-toluenesulfonyl
References
Alcaide B, Almendros P, Quirós MT, Fernández I (2013) Gold-catalyzed oxycyclization of allenic carbamates: expeditious synthesis of 1,3-oxazin-2-ones. Beilstein J Org Chem 9:818–826. https://doi.org/10.3762/bjoc.9.93
Ayed C, Palmier S, Lubin-Germain N et al (2010a) Indium-mediated alkynylation of sugars: synthesis of C-glycosyl compounds bearing a protected amino alcohol moiety. Carbohydr Res 345:2566–2570. https://doi.org/10.1016/j.carres.2010.07.033
Ayed C, Picard J, Lubin-Germain N et al (2010b) Synthesis of alkynes and alkynyl iodides bearing a protected amino alcohol moiety as functionalized amino acids precursors. Sci China Chem 53:1921–1926. https://doi.org/10.1007/s11426-010-4072-2
Badarau E, Suzenet F, Fînaru A-L, Guillaumet G (2009) Synthesis of 3-amino-8-azachromans and 3-amino-7-azabenzofurans via Inverse electron demand Diels–Alder reaction. Eur J Org Chem 2009:3619–3627. https://doi.org/10.1002/ejoc.200900191
Belanger D, Tong X, Soumare S et al (2009) Cyclic peptide-polymer complexes and their self-assembly. Chem Eur J. 15:4428–4436. https://doi.org/10.1002/chem.200802337 S4428/1-S4428/6
Benfodda Z, Bénimélis D, Reginato G, Meffre P (2015) Ethynylglycine synthon, a useful precursor for the synthesis of biologically active compounds: an update. Amino Acids 47:271–279. https://doi.org/10.1007/s00726-014-1902-0
Boibessot T, Bénimèlis D, Jean M et al (2016a) Synthesis of a novel rhizobitoxine-like triazole-containing amino acid. Synlett 27:2685–2688. https://doi.org/10.1055/s-0036-1588300
Boibessot T, Bénimélis D, Meffre P, Benfodda Z (2016b) Advances in the synthesis of α-quaternary α-ethynyl α-amino acids. Amino Acids 48:2081–2101. https://doi.org/10.1007/s00726-016-2276-2
Bolsakova J, Jirgensons A (2016) Synthesis of α-Ethynyl Glycines. Eur J Org Chem 2016:4591–4602. https://doi.org/10.1002/ejoc.201600253
Brummond KM, Yan B (2008) Rhodium(I)-catalyzed cycloisomerization reaction of yne-allenamides: an approach to cyclic enamides. Synlett 2008:2303–2308. https://doi.org/10.1055/s-2008-1078169
Cabarrocas G, Rafel S, Ventura M, Villalgordo J (2000a) A new approach toward the stereoselective synthesis of novel quinolyl glycines: synthesis of the enantiomerically pure Quinolyl-β-amino alcohol precursors. Synlett 2000:0595–0598. https://doi.org/10.1055/s-2000-6625
Cabarrocas G, Ventura M, Maestro M et al (2000b) Reaction between hydrazines and chiral α-acetylenic ketones: synthesis of novel enantiomerically pure pyrazolyl-β-amino alcohols†. Tetrahedron Asymmetry 11:2483–2493. https://doi.org/10.1016/S0957-4166(00)00204-4
Cabarrocas G, Ventura M, Maestro M et al (2001) Synthesis of novel optically pure quinolyl-β-amino alcohol derivatives from 2-amino thiophenol and chiral α-acetylenic ketones and their IBX-mediated oxidative cleavage to N-Boc quinolyl carboxamides. Tetrahedron Asymmetry 12:1851–1863. https://doi.org/10.1016/S0957-4166(01)00308-1
Callahan JF, Khatana SS, Bhatnagar PK (2000) Stereoselective synthesis of diaminosuberic acid via a chiral alkynyl oxazolidine. Synth Commun 30:1213–1219. https://doi.org/10.1080/00397910008087141
Cameron S, Khambay BPS (1998) Stereospecific synthesis of the amino acid, (S)-2-amino-(Z)-3,5-hexadienoic acid. Tetrahedron Lett 39:1987–1990. https://doi.org/10.1016/S0040-4039(98)00112-9
Chinchilla R, Nájera C (2007) The sonogashira reaction: a booming methodology in synthetic organic chemistry. Chem Rev 107:874–922. https://doi.org/10.1021/cr050992x
Chung JYL, Wasicak JT (1990) Synthesis of chiral α-acetylenic cyclic amines from α-amino acids: applications to differentially constrained oxotremorine analogues as muscarinic agents. Tetrahedron Lett 31:3957–3960. https://doi.org/10.1016/S0040-4039(00)94471-X
Comas-Barceló J, Harrity JPA (2017) Metal acetylides in cycloaddition reactions. Synthesis 49:1168–1181. https://doi.org/10.1055/s-0036-1588922
Crabbe P, Schlemper Elmer O, Kay Fair et al (1985) Allene Synthesis by organo-metallic reactions. Isr J Chem 26:147–151. https://doi.org/10.1002/ijch.198500085
Crisp GT, Jiang Y-L, Pullman PJ, De Savi C (1997) Elaboration of the side-chain of amino acid derivatives by palladium catalysed couplings. Tetrahedron 53:17489–17500. https://doi.org/10.1016/S0040-4020(97)10197-1
Dondoni A, Mariotti G, Marra A, Massi A (2001) Expeditious synthesis of β-linked glycosyl serine methylene isosteres (β-C-gly ser) via ethynylation of sugar lactones. Synthesis. https://doi.org/10.1055/s-2001-18058
Erdsack J, Krause N (2007) Synthesis of furanomycin derivatives by gold-catalyzed cycloisomerization of α-hydroxyallenes. Synthesis 2007:3741–3750. https://doi.org/10.1055/s-2007-990860
Evidente A, Kornienko A, Cimmino A et al (2014) Fungal metabolites with anticancer activity. Nat Prod Rep 31:617–627. https://doi.org/10.1039/C3NP70078J
Falorni M, Giacomelli G, Spanu E (1998) Synthesis of new α-amino- acids containing the isoxazole moiety. Tetrahedron Lett 39:9241–9244. https://doi.org/10.1016/S0040-4039(98)02010-3
Frydenvang K, Pickering DS, Greenwood JR et al (2010) Biostructural and pharmacological studies of bicyclic analogues of the 3-isoxazolol glutamate receptor agonist ibotenic acid. J Med Chem 53:8354–8361. https://doi.org/10.1021/jm101218a
Giacomelli G, De Luca L, Porcheddu A (2003) A method for generating nitrile oxides from nitroalkanes: a microwave assisted route for isoxazoles. Tetrahedron 59:5437–5440. https://doi.org/10.1016/S0040-4020(03)00859-7
Goswami K, Duttagupta I, Sinha S (2012a) Synthesis of optically active 2- and 3- indolylglycine derivatives and their oxygen analogues. J Org Chem 77:7081–7085. https://doi.org/10.1021/jo300708h
Goswami K, Paul S, Bugde ST, Sinha S (2012b) Synthesis of optically active homotryptophan and its oxygen and sulfur analogues. Tetrahedron 68:280–286. https://doi.org/10.1016/j.tet.2011.10.055
Goswami K, Chakraborty A, Sinha S (2013) Synthesis of optically active selenium-containing isotryptophan, homoiso-tryptophan, and homotryptophan. Eur J Org Chem 2013:3645–3647. https://doi.org/10.1002/ejoc.201300352
Govek SP, Overman LE (2007) Total synthesis of (+)-asperazine. Tetrahedron 63:8499–8513. https://doi.org/10.1016/j.tet.2007.05.127
Guillarme S, Plé K, Haudrechy A (2006) Selective synthesis of α-C-(Alkynyl)-galactosides by an efficient tandem reaction. J Org Chem 71:1015–1017. https://doi.org/10.1021/jo0519817
Holmberg P, Sohn D, Leideborg R et al (2004) Novel 2-aminotetralin and 3-aminochroman derivatives as selective serotonin 5-HT7 receptor agonists and antagonists. J Med Chem 47:3927–3930. https://doi.org/10.1021/jm0498102
Holmberg P, Tedenborg L, Rosqvist S, Johansson AM (2005) Novel 3-aminochromans as potential pharmacological tools for the serotonin 5-HT7 receptor. Bioorg Med Chem Lett 15:747–750. https://doi.org/10.1016/j.bmcl.2004.11.013
Huisgen R (1963) 1,3-Dipolar Cycloadditions. Past and Future. Angew Chem Int Ed Engl 2:565–598. https://doi.org/10.1002/anie.196305651
Katagiri K, Tori K, Kimura Y et al (1967) A new antibiotic. furanomycin, an isoleucine antagonist. J Med Chem 10:1149–1154. https://doi.org/10.1021/jm00318a035
Kavitha M, Mahipal B, Mainkar PS, Chandrasekhar S (2011) Click reaction on in situ generated β-azidostyrenes from cinnamic acid using CAN–NaN3: synthesis of N-styryl triazoles. Tetrahedron Lett 52:1658–1662. https://doi.org/10.1016/j.tetlet.2011.01.129
Khutorianskyi A, Chalyk B, Borysko P et al (2017) Difluoromethyl nitrile oxide (CF2HCNO): a neglected chemical reagent. Eur J Org Chem 2017:3935–3940. https://doi.org/10.1002/ejoc.201700764
Kumar P, Shukhman D, Laughlin ST (2016) A photocaged, cyclopropene-containing analog of the amino acid neurotransmitter glutamate. Tetrahedron Lett 57:5750–5752. https://doi.org/10.1016/j.tetlet.2016.10.106
Lin H, Kazmaier U (2007) Regioselective mo-catalyzed hydrostannations as key steps in the synthesis of functionalized amino alcohols and heterocycles. Eur J Org Chem 2007:2839–2843. https://doi.org/10.1002/ejoc.200700126
Mallampudi NA, Reddy GS, Maity S, Mohapatra DK (2017) Gold(I)-Catalyzed Cyclization for the Synthesis of 8-Hydroxy-3—substituted Isocoumarins: total Synthesis of Exserolide F. Org Lett 19:2074–2077. https://doi.org/10.1021/acs.orglett.7b00673
Mazuela J, Antonsson T, Johansson MJ et al (2017) Direct Synthesis of N-Alkyl Arylglycines by Organocatalytic Asymmetric Transfer Hydrogenation of N-Alkyl Aryl Imino Esters. Org Lett. https://doi.org/10.1021/acs.orglett.7b02627
Meffre P, Le Goffic F (1996) β, γ-Alkynylα-amino acids: a synthetic challenge. Amino Acids 11:313–328. https://doi.org/10.1007/BF00807939
Meffre P, Gauzy L, Branquet E et al (1996) Synthesis of optically active β, γ-alkynylglycine derivatives. Tetrahedron 52:11215–11238. https://doi.org/10.1016/0040-4020(96)00630-8
Ohno H, Ando K, Hamaguchi H et al (2002) A highly cis-selective synthesis of 2-ethynylaziridines by intramolecular amination of chiral bromoallenes: improvement of stereoselectivity based on the computational investigation. J Am Chem Soc 124:15255–15266. https://doi.org/10.1021/ja0262277
Owens LD, Guggenheim S, Hilton JL (1968) Rhizobium-synthesized phytotoxin: an inhibitor of β-cystathionase in Salmonella typhimurium. Biochim Biophys Acta BBA Gen Subj 158:219–225. https://doi.org/10.1016/0304-4165(68)90134-7
Pulley SR, Sen S, Vorogushin A, Swanson E (1999) Diaryl ethers using fischer chromium carbene mediated benzannulation. Org Lett 1:1721–1723. https://doi.org/10.1021/ol990949u
Pulley SR, Czakó B, Brown GD (2005) Synthesis of arylglycines via the Dötz benzannulation reaction. Tetrahedron Lett 46:9039–9042. https://doi.org/10.1016/j.tetlet.2005.10.105
Raji Reddy C, Krishna G, Kavitha N et al (2012) Access to 2,3-disubstituted benzofurans through one-pot acid-catalyzed nucleophilic substitution/TBAF-mediated oxacycloisomerization. Eur J Org Chem 2012:5381–5388. https://doi.org/10.1002/ejoc.201200708
Reginato G, Mordini A, Degl’Innocenti A, Caracciolo M (1995) Stereoselective synthesis of (R)-(−)-2,2-dimethyl-3-t-butoxycarbonyl-4-ethynyl-oxazolidine: a chiral building block for the synthesis of a new class of substituted alkynes. Tetrahedron Lett 36:8275–8278. https://doi.org/10.1016/0040-4039(95)01725-w
Reginato G, Mordini A, Caracciolo M (1997) Synthetic Elaboration of the Side Chain of (R)-2,2-dimethyl-3-(tert-butoxycarbonyl)-4-ethynyloxazolidine: a new regio—and stereoselective strategy to δ-functionalized β-amino alcohols. J Org Chem 62:6187–6192. https://doi.org/10.1021/jo970619s
Reginato G, Mordini A, Valacchi M (1998) A stereoselective approach to the synthesis of γ-silylated amino acids. Tetrahedron Lett 39:9545–9548. https://doi.org/10.1016/S0040-4039(98)02120-0
Reginato G, Mordini A, Valacchi M, Grandini E (1999) Silylcupration of (R)-2,2-Dimethyl-3-(tert-butoxycarbonyl)-4-ethynyloxazolidine: a stereoselective approach to the synthesis of γ-silylated saturated and unsaturated α-amino acids. J Org Chem 64:9211–9216. https://doi.org/10.1021/jo991272r
Reginato G, Mordini A, Verrucci M et al (2000) A new approach to non racemic saturated and unsaturated 5-aminoalkyl methyl ketones. Tetrahedron Asymmetry 11:3759–3768. https://doi.org/10.1016/S0957-4166(00)00335-9
Reginato G, Gaggini F, Mordini A, Valacchi M (2005a) Stereoselective synthesis of dienylamines: from amino acids to E-alkene dipeptide isosters. Tetrahedron 61:6791–6800. https://doi.org/10.1016/j.tet.2005.04.068
Reginato G, Meffre P, Gaggini F (2005b) Ethynylglycine synthon from Garner’s aldehyde: a useful precursor for the synthesis of non-natural amino acids. Amino Acids 29:81–87. https://doi.org/10.1007/s00726-005-0184-y
Reginato G, Mordini A, Meffre P et al (2006) New unsaturated amino acids containing an allylsilane moiety on the lateral chain. Tetrahedron Asymmetry 17:922–926. https://doi.org/10.1016/j.tetasy.2006.02.017
Röhrig UF, Awad L, Grosdidier A et al (2010) Rational design of indoleamine 2,3-dioxygenase inhibitors. J Med Chem 53:1172–1189. https://doi.org/10.1021/jm9014718
Serrat X, Cabarrocas G, Rafel S et al (1999) A highly efficient and straightforward stereoselective synthesis of novel chiral α-acetylenic ketones. Tetrahedron Asymmetry 10:3417–3430. https://doi.org/10.1016/S0957-4166(99)00357-2
Spangenberg T, Schoenfelder A, Breit B, Mann A (2010) 1,2-Diastereoselective C–C bond-forming reactions for the synthesis of chiral β-branched α-amino acids. Eur J Org Chem 2010:6005–6018. https://doi.org/10.1002/ejoc.201000865
Stecko S, Mames A, Furman B, Chmielewski M (2009) Asymmetric kinugasa reaction of cyclic nitrones and nonracemic acetylenes. J Org Chem 74:3094–3100. https://doi.org/10.1021/jo900121x
Sugawara M, Okazaki S, Nukui N et al (2006) Rhizobitoxine modulates plant–microbe interactions by ethylene inhibition. Biotechnol Adv 24:382–388. https://doi.org/10.1016/j.biotechadv.2006.01.004
Totobenazara J, Burke AJ (2015) New click-chemistry methods for 1,2,3-triazoles synthesis: recent advances and applications. Tetrahedron Lett 56:2853–2859. https://doi.org/10.1016/j.tetlet.2015.03.136
Usuki T, Yamada H, Hayashi T et al (2012) Total synthesis of COPD biomarker desmosine that crosslinks elastin. Chem Commun 48:3233–3235. https://doi.org/10.1039/C2CC17958J
von Nussbaum F, Brands M, Hinzen B et al (2006) Antibacterial Natural Products in Medicinal Chemistry—Exodus or Revival? Angew Chem Int Ed 45:5072–5129. https://doi.org/10.1002/anie.200600350
Wzorek JS, Knöpfel TF, Sapountzis I, Evans DA (2012) A macrocyclic approach to tetracycline natural products. investigation of transannular alkylations and michael additions. Org Lett 14:5840–5843. https://doi.org/10.1021/ol302691j
Xiong K, Fuhrmann JJ (1996) Comparison of rhizobitoxine-induced inhibition of β-cystathionase from different bradyrhizobia and soybean genotypes. Plant Soil 186:53–61. https://doi.org/10.1007/BF00035055
Yamada H, Hayashi T, Usuki T (2015) Total synthesis of the COPD biomarker desmosine via stepwise sonogashira Cross-coupling reactions. Bull Chem Soc Jpn 88:673–683. https://doi.org/10.1246/bcsj.20140394
Yamakawa T, Ideue E, Shimokawa J, Fukuyama T (2010) Total synthesis of tryprostatins A and B. Angew Chem Int Ed 49:9262–9265. https://doi.org/10.1002/anie.201004963
Yamakawa T, Ideue E, Iwaki Y et al (2011) Total synthesis of tryprostatin A and B. Tetrahedron 67:6547–6560. https://doi.org/10.1016/j.tet.2011.05.112
Yamakawa T, Ideue E, Shimokawa J, Fukuyama T (2014) Corrigendum: total synthesis of tryprostatins A and B. Angew Chem Int Ed 53:8808–8808. https://doi.org/10.1002/anie.201401055
Yamashita Y, Saito Y, Imaizumi T, Kobayashi S (2014) A Lewis acid/metal amide hybrid as an efficient catalyst for carbon-carbon bond formation. Chem Sci 5:3958–3962. https://doi.org/10.1039/C4SC01332H
Yanada R, Obika S, Kobayashi Y et al (2005) Stereoselective synthesis of 3-alkylideneoxindoles using tandem indium-mediated carbometallation and palladium-catalyzed cross-coupling reactions. Adv Synth Catal 347:1632–1642. https://doi.org/10.1002/adsc.200505147
Yasuta T, Satoh S, Minamisawa K (1999) New assay for rhizobitoxine based on inhibition of 1-aminocyclopropane-1-carboxylate synthase. Appl Environ Microbiol 65:849–852
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Research subjects
This review is a compilation of the previous works performed by different authors. No animal or human was used or harmed in this work.
Informed consent
This manuscript is being submitted after consent was obtained from all authors, and all authors are aware of this manuscript submission.
Additional information
Handling Editor: J. D. Wade.
Rights and permissions
About this article
Cite this article
Benfodda, Z., Benimélis, D., Reginato, G. et al. Ethynylglycine synthon, a useful precursor for the synthesis of biologically active compounds: an update. Part II: synthetic uses of ethynylglycine synthon. Amino Acids 50, 1307–1328 (2018). https://doi.org/10.1007/s00726-018-2628-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-018-2628-1