Abstract
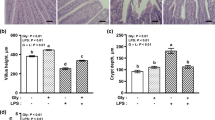
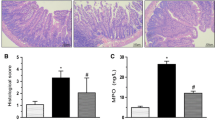
This study investigated the nitric oxide (NO) role as a mediator of arginine on bacterial translocation (BT) and gut damage in mice after intestinal obstruction (IO). The effects of pretreatment with arginine with or without NO inhibition on the systemic and local immunological response were also assessed. Mice were categorized into four groups. Group ARG received chow containing 2 % arginine, while group ARG + l-NAME received the same diet plus l-NAME (N-nitro-l-arginine methyl ester) by gavage. The IO and Sham groups were fed standard chow. After 7 days, animals were gavaged with radiolabeled Escherichia coli, anesthetized and subjected to IO, except the Sham group. Animals were euthanized after 18 h, and BT was evaluated in the mesenteric lymph nodes, blood, liver, spleen and lungs. In another experiment, the intestinal injury was assessed regarding intestinal permeability and ileum histological analyses. Intestinal secretory immunoglobulin A (sIgA) levels, serum IFN-γ and IL-10 cytokines were assessed. Arginine reduced BT, but NO inhibition enhanced BT compared with the ARG group (p < 0.05). Intestinal permeability in the ARG and ARG + l-NAME groups was similar but decreased when compared with the IO group (p < 0.05). Histological preservation was observed. Arginine treatment increased IL-10 and sIgA levels when compared with the Sham and IO groups (p < 0.05). The cytokines and sIgA concentrations were similar in the ARG + l-NAME and Sham groups. Arginine appeared to reduce BT and its effects on the modulation of cytokines and secretory IgA in mice after IO are mediated by NO production.




Similar content being viewed by others
References
Benbernou N, Esnault S, Shin HC, Fekkar H, Guenounou M (1997) Differential regulation of IFN-gamma, IL-10 and inducible nitric oxide synthase in human T cells by cyclic AMP-dependent signal transduction pathway. Immunology 91:361–368
Biancofiore G, Bindi L, Miccoli M, Metelli MR, Panicucci E, Baggiani A, Filipponi F (2013) Balance of pro- and anti-inflammatory cytokines in cirrhotic patients undergoing liver transplantation. Transpl Immunol 28:193–197
Brayden DJ, Jepson MA, Baird AW (2005) Intestinal Peyer’s patch M cells and oral vaccine targeting. Drug Discov Today 10:1145–1157
Clayburgh DR, Shen L, Turner JR (2004) A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest 84:282–291
Demirkiran AE, Balkaya M, Tuncyurek P, Cevikel MH, Culhaci N, Iyigor M, Ozgun H, Aydin N, Boylu S (2006) The effects of nitric oxide supplementation and inhibition on bacterial translocation in bile duct ligated rats. Acta Chir Belg 106:202–205
Ding L, Li J, Li Y, Zhu N, Liu F, Tan L (2004) Intestinal barrier damage caused by trauma and lipopolysaccharide. World J Gastroenterol 10:2373–2378
Diniz SOF, Resende BM, Nunan EA, Simal CJR, Cardoso VN (1999) 99mTechnetium labelled Escherichia coli. Appl Radiat Isto 51:33–36
Du Plessis J, Vanheel H, Janssen CEI, Roos L, Slavik T, Stivaktas PI, Nieuwoudt M, Wyk SGV, Vieira W, Pretorius E, Beukes M, Farré R, Tack J, Laleman W, Fevery J, Nevens F, Roskams T, Van der Merwe SW (2013) Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J Hepatol 58:1125–1132
Ersin S, Tuncyurek P, Esassolak M, Alkanat M, Buke C, Yilmaz M, Telefonuc A, Kose T (2000) The prophylactic and therapeutic effects of glutamine and arginine enriched diets on radiation induced enteritis in rats. J Surg Res 89:121–125
Fan J, Meng Q, Guo G, Xie Y, Li X, Xiu Y, Li T, Ma L (2010) Effects of early enteral nutrition supplemented with arginine on intestinal mucosal immunity in severely burned mice. Clin Nutr 29:124–130
Hajishengallis G, Nikolova E, Russell MW (1992) Inhibition of Streptococcus mutans adherence to saliva-coated hydroxyapatite by human secretory immunoglobulin A (S-IgA) antibodies to cell surface protein antigen I/II: reversal by IgA1 protease cleavage. Infect Immun 60:5057–5064
Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U (2001) Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA 286:944–953
Ibiza S, Serrador JM (2008) The role of nitric oxide in the regulation of adaptive immune responses. Inmunología 27:103–117
Izcue A, Coombes JL, Powrie F (2009) Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol 27:313–338
Kobayashi T, Yamamoto M, Hiroi T, Mcghee J, Takeshita Y, Kiyono H (1998) Arginine enhances induction of T helper 1 and T helper 2 cytokine synthesis by Peyer’s patch alpha beta T cells and antigen-specific mucosal immune response. Biosci Biotechnol Biochem 62:2334–2340
Koppelmann T, Pollak Y, Mogilner J, Bejar J, Coran AG, Sukhotnik I (2012) Dietary l-arginine supplementation reduces methotrexate-induced intestinal mucosal injury in rat. BMC Gastroenterol 12:41
Kung SP, Wu CW, Lui WY (2001) Arginine modulated cyclosporine induced immune suppression in rats transplanted with gastric cancer cells. In Vivo 15:39–44
Manzanares W, Heyland DK (2012) Pharmaconutrition with arginine decreases bacterial translocation in an animal model of severe trauma. Is a clinical studied justified? The time is now! Crit Care Med 40:350–352
Miki S, Takeyama N, Tanaka T, Nakatani T (2005) Immune dysfunction in endotoxicosis: role of nitric oxide produced by inducible nitric oxide synthase. Crit Care Med 33:716–720
Moinard C, Barbar S, Choisy C, Butel MJ, Bureau MF, Hasselmann M, Cynober L (2012) Arginine reduces bacterial invasion in rats with head injury: an in vivo evaluation by bioluminescence. Crit Care Med 40:278–280
Nadler EP, Ford HR (2000) Regulation of bacterial translocation by nitric oxide. Pediatr Surg Int 16:165–168
Ochoa JB, Strange J, Kearney P, Gellin G, Endean E, Fitzpatrick E (2001) Effects of l-arginine on the proliferation of T lymphocyte subpopulations. JPEN 25:23–29
Osowska S, Neveux N, Nakib S, Lasserre V, Cynober L, Moinard C (2008) Impairment of arginine metabolism in rats after massive intestinal resection: effect of parenteral nutrition supplemented with citrulline compared with arginine. Clin Sci(Lond) 115(5):159–166
Quirino IEP, Correia MITD, Cardoso VN (2007) The impact of arginine on bacterial translocation in an intestinal obstruction model in rats. Clin Nutr 26:335–340
Rhoads JM, Wu G (2009) Glutamine, arginine and leucine signaling in the intestine. Amino Acids 37:111–122
Rodrigues ACP, Cara DC, Fretez SHG, Cunha FQ, Vieira EC, Nicoli JR (2000) Saccharomyces boulardii stimulates sIgA production and the phagocytic system of gnotobiotic mice. J Appl Microbiol 89:404–414
Roozendaal R, Vellenga E, Postma DS, De Monchy JG, Kauffman HF (1999) Nitric oxide selectively decreases interferon gamma expression by activated human T lymphocytes via a cGMP independent mechanism. Immunology 98:393–399
Samel S, Keese M, Laning S, Kleczka M, Gretz N, Hafner M, Sturm J, Post S (2003) Supplementation and inhibition of nitric oxide synthesis influences bacterial transit time during bacterial translocation in rats. Shock 19:378–382
Shanahan F (2002) The host–microbe interface within the gut. Best Pract Res Clin Gastroenterol 16:915–930
Shang HF, Wang YY, Lai YN, Chiu WC, Yeh SL (2004) Effects of arginine supplementation on mucosal immunity in rats with septic peritonitis. Clin Nutr 23:561–569
Sukhotnik I, Mogilner J, Krausz MM, Lurie M, Hirsh M, Coran AG, Shiloni E (2004) Oral arginine reduces gut mucosal injury caused by lipopolysaccharide in rat. J Surg Res 122:256–262
Tanaka A, Mizoguchi H, Kunikata T, Miyazawa T, Takeuchi K (2001) Protection by constitutively formed nitric oxide of intestinal damage induced by indomethacin in rats. J Physiol 95:35–41
Van Der Veen R (2001) Nitric oxide and T helper cell immunity. Int Immunopharmacol 1:1491–1500
Viana ML, Santos RG, Generoso SV, Arantes RME, Correia MITD, Cardoso VN (2010) Pretreatment with arginine preserves intestinal barrier integrity and reduces bacterial translocation in mice. Nutrition 26:218–223
Wiley JW (2007) The many faces of nitric oxide: cytotoxic, cytoprotective or both. Neurogastroenterol Motil 19:541–544
Wu L, Chiu H, Peng W, Lin B, Lu K, Lu Y, Yu LC (2011) Epithelial inducible nitric oxide synthase causes bacterial translocation by impairment of enterocytic tight junctions via intracellular signals of Rho-associated kinase and protein kinase C zeta. Crit Care Med 39:2087–2098
Zulfikaroglu B, Zufikaroglu E, Ozmen MM, Berkem R, Erdogan S, Besler HT, Koc M, Korkmaz A (2003) The effect of immunonutrition on bacterial translocation, and intestinal villus atrophy in experimental obstructive jaundice. Clin Nutr 22:277–281
Acknowledgments
The study was supported by grants from Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Conselho Nacional de Pesquisa (CNPq).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Viana, M.L., dos Santos, R.d.G.C., Generoso, S.d.V. et al. The role of l-arginine-nitric oxide pathway in bacterial translocation. Amino Acids 45, 1089–1096 (2013). https://doi.org/10.1007/s00726-013-1558-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-013-1558-1