Summary
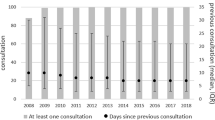
Age-related macular degeneration (AMD) is the leading cause of blindness in patients over 50 years of age in developed countries. It is estimated that the projected number of people with AMD will be 196 million worldwide by the year 2020, increasing to 288 million by 2040. Of these patients, 10–20% suffering from the fast progressing neovascular form of the disease (nAMD) account for 90% of all cases of severe vision loss. In fact, AMD is responsible for 8.7% of all cases of blindness worldwide. These numbers indicate the substantial burden of the disease. The WHO estimates that 246 million people worldwide currently have low vision and 39 million are blind. A literature (Medline) and Internet research was performed to better understand the prevalence of AMD and how health care could be prepared to cope with it in the future. In 2015, there were 90,010 intravitreal injections (IVI) in Austrian hospitals and primary care units. In 2016, this number rose to >100,000 IVI as the applications increase by approx. 15–20% every year. Since health insurances do not refund IVI in primary care, these services are channeled toward clinical wards, causing overcrowded waiting rooms and dissatisfied patients. Despite this high number of IVI in Austria, real-life data show that the number of IVI given today is not sufficient to keep visual acuity on a steady level. Therefore, new and long-acting treatment options are needed to end the burden for clinics and patients and to increase treatment efficiency by simplified protocols. Herein, a potential new gene-therapeutic approach using nonviral vectors and somatic pigment epithelial cells to overcome the imbalance of pigment epithelium-derived factor and vascular endothelial growth factor in nAMD is described.
Zusammenfassung
Die altersbedingte Makuladegeneration (AMD) ist die Hauptursache der Erblindung bei Patienten über 50 Jahren in Industrieländern. Es wird geschätzt, dass die Zahl der Personen mit AMD im Jahr 2020 voraussichtlich auf 196 Mio. weltweit ansteigen wird und bis 2040 auf 288 Mio. Von dieser Population leiden 10–20 % an der schnell fortschreitenden neovaskulären Form der Krankheit (nAMD), die aber 90 % aller Fälle mit schwerem Sehverlust ausmachen. AMD ist die Ursache für 8,7 % aller Erblindungen weltweit. Diese Zahlen zeigen die substanzielle Belastung durch die Erkrankung. Laut Weltgesundheitsorganisation (WHO) sind derzeit 39 Mio. blind, und 246 Mio. leiden unter schlechter Sehkraft. Für ein besseres Verständnis der Prävalenz der AMD und zur Prüfung, ob das Gesundheitssystem auf den zukünftigen Patientenanstieg vorbereitet ist, wurde eine Literatur- (Medline) und Internetrecherche durchgeführt. Es gab 2015 in österreichischen Klinikambulanzen und im niedergelassenen Bereich 90.010 intravitreale operative Medikationseinspritzungen (IVOM). Diese Zahl überstieg 2016 die 100.000er-Grenze, da IVOM jährlich um etwa 15–20 % ansteigen. Da die Sozialversicherungen IVOM im niedergelassenen Bereich nicht erstatten, wird für diesen Service an die Klinikambulanzen verwiesen, was überfüllte Wartesäle und unzufriedene Patienten zur Folge hat. Trotz der hohen Zahl der IVOM allein in Österreich zeigen reale Daten, dass die Anzahl der IVOM nicht zum Visuserhalt ausreicht. Deshalb werden neue und langwirksame Behandlungsoptionen benötigt, um die Belastung für Klinik und Patient zu senken und die Wirksamkeit durch einfache Therapieprotokolle zu erhöhen. In der vorliegenden Arbeit wird ein wirksames neues Gentherapieverfahren mittels nichtviraler Vektoren und somatischer Pigmentepithelzellen vorgestellt, welches das Ungleichgewicht von „pigment epithelium-derived factor“ (PEDF) und „vascular endothelial growth factor“ (VEGF) bei nAMD beheben soll.



Similar content being viewed by others
References
Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e16.
WHO. Visual impariment and blindness 2014. http://www.who.int/mediacentre/factsheets/fs282/en/. Accessed 9 May 2016.
Statistik Austria. Anzahl der unterschiedlichen medizinischen Einzelleistungen bei Spitalsentlassungen 2009 bis 2014. http://www.statistik.at/web_de/nomenu/suchergebnisse/index.html.
Holz FG, Helb HM, Bindewald-Wittich A, Scholl HPN. Modern pharmacotherapy of age-related macular degeneration. Internist. 2006;47(2):192–8.
Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet Lond Engl. 2012;379(9827):1728–38.
Bauer P, Barthelmes D, Kurz M, Fleischhauer JC, Sutter FK. The potential effect of population development, smoking and antioxidant supplementation on the future epidemiology of age-related macular degeneration in Switzerland. Klin Monbl Augenheilkd. 2008;225(5):376–9.
Schmucker Ch, Antes G, Leglemann M, Schmacke N, Ehlken Ch, Agostini H‑J. et al. Therapy of age-related macular degeneration - evidence report. 2009. unpublished.
Österreichische Ophthalmologische Gesellschaft. 2016. http://www.augen.at/patienteninformation/altersbedingte_makuladegeneration.php. Accessed 9 Apr 2016.
netdoktor. AMD Ambulanzen und Artikel zur altersbedingten Makuladegeneration. http://www.netdoktor.at/ambulanz/amd-279558.
Boulanger-Scemama E, Querques G, About F, Puche N, Srour M, Mane V, et al. Ranibizumab for exudative age-related macular degeneration: a five year study of adherence to follow-up in a real-life setting. J Fr Ophtalmol. 2015;38(7):620–7.
Kieselbach G, Vavrovsky A, Hochreiter R. IVOM in Österreich – eine Auswertung anhand realer Patientenzahlen. Spektrum Augenheilkd. 2016;30:106–10.
CATT Research Group, Martin DF, Maguire MG, Ying G, Grunwald JE, Fine SL, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–908.
Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Cortez R, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99(2):220–6.
Holz FG, Schmitz-Valckenberg S, Fleckenstein M. Recent developments in the treatment of age-related macular degeneration. J Clin Invest. 2014;124(4):1430–8.
Cohen SY, Mimoun G, Oubraham H, Zourdani A, Malbrel C, Queré S, et al. Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: the LUMIERE study. Retina. 2013;33(3):474–81.
Ziemssen F, Eter N, Fauser S, Bopp S, Radermacher M, Hasanbasic Z, et al. Retrospective investigation of anti-VEGF treatment reality and effectiveness in patients with neovascular age-related macular degeneration (AMD) in Germany: treatment reality of ranibizumab for neovascular AMD in Germany. Ophthalmologe. 2015;112(3):246–54.
Stewart MW. Pharmacokinetics, pharmacodynamics and pre-clinical characteristics of ophthalmic drugs that bind VEGF. Expert Rev Clin Pharmacol. 2014;7(2):167–80.
Meyer CH, Krohne TU, Charbel Issa P, Liu Z, Holz FG. Routes for drug delivery to the eye and retina: Intravitreal injections. Dev Ophthalmol. 2016;55:63–70.
Krohne TU, Holz FG, Meyer CH. Pharmacokinetics of intravitreally administered VEGF inhibitors. Ophthalmologe. 2014;111(2):113–20.
Beck M, Munk MR, Ebneter A, Wolf S, Zinkernagel MS. Retinal ganglion cell layer change in patients treated with anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Am J Ophthalmol. 2016;167:10–7.
Grunwald JE, Daniel E, Huang J, Ying G‑S, Maguire MG, Toth CA, et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(1):150–61.
Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2‑year findings of the IVAN randomised controlled trial. Lancet. 2013;382(9900):1258–67.
Bhisitkul RB, Mendes TS, Rofagha S, Enanoria W, Boyer DS, Sadda SR, et al. Macular atrophy progression and 7‑year vision outcomes in subjects from the ANCHOR, MARINA, and HORIZON studies: the SEVEN-UP study. Am J Ophthalmol. 2015;159(5):915–924.e2.
Matamoros E, Maurel F, Léon N, Solomiac A, Bardoulat I, Joubert M, et al. Quality of life in patients suffering from active exudative age-related macular degeneration: the EQUADE study. Ophthalmologica. 2015;234(3):151–9.
Thumann G, Kropp M, Harmening N. Transposon-based, targeted ex vivo gene therapy to treat age-related macular degeneraion (AMD): the TargetAMD project. Poster presentation at the annual meeting of ESCGT 2016 in Florence, Italy.
Kropp M, Chronopoulos A, Conti A. Results of a biodistribution study of Venus tgransfected pigment epithelial cells transplnated subretinally in rabbits. Poster presentation at the annual meeting of ESGCT 2016 in Florence, Italy.
Harmening N, Sealy G, Johnen S. Translation of GLP-grade electroporation of primary pigment epithelial cells to GMP-grade GTMP manufacturing for clinical use. Poster presentation at the annual meeting of ESGCT 2016 in Florence, Italy.
European Parliament, European Council. Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC. Off J Eur Union. 2014 May 27 (L158):1–76.
European Commission. Directorate – General for Health and Food Safety. Implementation measures by the Commission in the context of regulation (EU) No. 536/2014 – overview and state of play. Brussels: European Commission; 2014.
Fernández-Robredo P, Sancho A, Johnen S, Recalde S, Gama N, Thumann G, et al. Current treatment limitations in age-related macular degeneration and future approaches based on cell therapy and tissue engineering. J Ophthalmol. 2014;2014:ID510285.
Schauwvlieghe AME, Dijkman G, Hooymans JM, Verbraak FD, Hoyng CB, Dijkgraaf MGW, et al. Comparing the effectiveness of bevacizumab to ranibizumab in patients with exudative age-related macular degeneration. The BRAMD study. PLOS ONE. 2016;11(5):e0153052.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
U. Stolba, S. Ansari-Shahrezaei, S. Hagen, M. Stattin, S. Schmid, M. Kropp, N. Harmening, G. Thumann, and TargetAMDGroup declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Stolba, U., Ansari-Shahrezaei, S., Hagen, S. et al. Neovascular age-related macular degeneration in Austria. Spektrum Augenheilkd. 31, 206–211 (2017). https://doi.org/10.1007/s00717-017-0356-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00717-017-0356-7
Keywords
- Age-related macular degeneration
- Electroporation
- Ocular gene therapy
- Gene therapy medicinal product
- GTMP