Abstract
The outbreak of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a serious threat to global public health. The emergence of SARS-CoV-2 variants is a significant concern regarding the continued effectiveness of vaccines and antiviral therapeutics. Thus, natural products such as foods, drinks, and other compounds should be investigated for their potential to treat COVID-19. Here, we examined the in vitro antiviral activity against SARS-CoV-2 of various polyethylene terephthalate (PET)-bottled green Japanese teas and tea compounds. Six types of PET-bottled green tea were shown to inhibit SARS-CoV-2 at half-maximal inhibitory concentrations (IC50) of 121- to 323-fold dilution. Our study revealed for the first time that a variety of PET-bottled Japanese green tea drinks inhibit SARS-CoV-2 infection in a dilution-dependent manner. The tea compounds epigallocatechin gallate (EGCG) and epicatechin gallate showed virucidal activity against SARS-CoV-2, with IC50 values of 6.5 and 12.5 µM, respectively. The investigated teas and tea compounds inactivated SARS-CoV-2 in a dose-dependent manner, as demonstrated by the viral RNA levels and infectious titers. Furthermore, the green teas and EGCG showed significant inhibition at the entry and post-entry stages of the viral life cycle and inhibited the activity of the SARS-CoV-2 3CL-protease. These findings indicate that green tea drinks and tea compounds are potentially useful in prophylaxis and COVID-19 treatment.
Similar content being viewed by others
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19) with a high potential for transmissibility between humans and was first reported in Wuhan, China. The World Health Organization (WHO) declared this viral infection to be a public health emergency of international concern on January 30, 2020 [1]. As of December 29, 2021, the WHO disease situation dashboard showed that more than 281 million confirmed cases and more than 5 million deaths have been recorded in more than 220 countries. Infection with SARS-CoV-2 can lead to a wide range of clinical presentations, from asymptomatic infection in 1% of laboratory-confirmed cases, to mild, severe, and critical infections in 81%, 14%, and 5% of symptomatic cases, respectively [2]. The betacoronavirus SARS-CoV-2 (family Coronaviridae, order Nidovirales) is an enveloped, positive-sense, single-stranded RNA virus. Infection is initiated by the binding of virus spike glycoprotein to its receptor, angiotensin-converting enzyme 2 (ACE2), on the cell membrane [3]. ACE2 is found in several tissue and cell types, including alveolar lung cells, gastrointestinal tissue, and even the brain [4], and SARS-CoV-2 also affects these organs.
Efficacious vaccines are being applied worldwide, and 54.8% of the world population has received at least one dose of a COVID-19 vaccine [5]. Although a large fraction of the global population remains unvaccinated, the expectation of a continuously mutating virus that may come to exhibit at least partial resistance to vaccines emphasizes that drug development must proceed [6]. Clinical investigations have focused on several approved antiviral drugs, including remdesivir [7] and molnupiravir [8]. To mitigate the SARS-CoV-2 pandemic, effective measures should be taken to control infection. To reduce the transmission of SARS-CoV-2, protective actions have been implemented to encourage public health, including regular hand washing, covering coughs, imposing lockdowns, enacting social distancing measures, and encouraging safe food practices. Natural products with antiviral activity would be useful for reducing the spread of viruses in the population, as they are inexpensive and easily obtained and used. Some foods (including those derived from plants, animals, and fungi) have antiviral and immune-boosting properties that can protect against numerous viral infections, including coronavirus and other RNA virus infections [9]. Several natural products, such as polysaccharides from seaweed, abalone viscera, citrus fruits, black chokeberry juice, and polyphenolic compounds in tea and green tea herbal substances, have an antiviral effect against SARS-CoV-2 [10,11,12,13,14].
Several studies have shown that tea has health-promoting properties and is protective against cancer, diabetes, and neurological, cardiovascular, metabolic, and infectious diseases [11]. Black tea is the most-consumed tea worldwide, followed by green tea, oolong tea, and white tea; these teas have shown beneficial effects in humans [15]. The catechin polyphenol compounds present in green tea include (-) epigallocatechin gallate (EGCG) and (-) epicatechin gallate (ECG). Teas bottled in polyethylene terephthalate (PET) bottles offer advantages such as being lightweight, easily portable, sturdy, widely available, and able to maintain product integrity. Here, we evaluated the antiviral effect of various Japanese PET-bottled green teas and tea compounds (EGCG, ECG) against SARS-CoV-2.
Materials and methods
Virus and cells
SARS-CoV-2 (TY/WK-521, Japan, 2020) was provided by the National Institute of Infectious Disease, Japan, and was propagated in Vero-E6 cells (African green monkey kidney cells) provided by ATCC, Ltd. and cultured in minimum essential medium (MEM) supplemented with 10% fetal calf serum (FCS). The supernatant was harvested 5 days after infection, centrifuged at 2,500 rpm for 10 min, and stored at -80 °C as virus stocks. All experiments using infectious SARS-CoV-2 were performed in a biosafety level 3 (BSL3) laboratory at Nagasaki University according to standard BSL3 guidelines.
Materials
Six brands of bottled Japanese green tea, green tea beverage (GTB)-A to -F, were purchased from vending machines, supermarkets, and convenience stores in Japan. The biochemical compounds present in green tea (EGCG, ECG) were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan).
Evaluation of antiviral activity
One day prior to infection, Vero E6 cells were seeded into 96-well plates. The cells were treated with diluted tea or the compounds for 1 h, after which the virus was added at a multiplicity of infection (MOI) of 0.02. The cells were then incubated for 2 h to allow infection, and the inoculum was removed. The cells were washed with phosphate-buffered saline (PBS), and fresh tea- or compound-containing medium was added, after which the cells were incubated further until 2 days postinfection (p.i.). Virus in the infected cell supernatant was quantified using quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR). Remdesivir- and virus-containing medium was used as a positive and negative control (virus only), respectively.
Time-of-addition experiment
To evaluate the effects of teas and compounds on the entry or post-entry steps of the SARS-CoV-2 life cycle, a time-of-addition assay was performed. The time-of-addition studies were performed using Vero E6 cells grown in 96-well plates. For experiments in which the inhibitor was present at all stages of infection (full treatment), cells were pre-treated with the teas or compounds for 1 h prior to viral infection and infected with the virus for 2 h (MOI 0.1). The supernatant containing a mixture of the virus and tea or compound was then removed, and the cells were washed with PBS. The cells were then incubated with tea or compound in fresh medium until 16 h p.i. To test the effect of inhibitors of viral entry, the procedure was the same as above except that the cells were incubated without tea or compound until 16 h p.i. For post-entry treatment, the cells were infected with the virus for 2 h, after which the cells were washed and incubated in fresh medium containing tea or compound until 16 h p.i. Dimethyl sulfoxide (DMSO)-treated cells were included as a control in all experiments. Cell lysates were collected for western blot (WB) analysis.
Viral RNA extraction and quantitative real-time RT-PCR
For viral RNA extraction, 100 mL of infected cell supernatant was harvested using a Nextractor NX-48 robot and an NX-48S Viral NA Kit (Genolution Inc., Seoul, South Korea) according to the manufacturer’s instructions. A volume of 5 μL of RNA was used for qRT-PCR, and amplification of the nucleocapsid (N) gene was performed using 20 µL of reaction mixture consisting of 5 μL of TaqMan master mix, 7 µL of nuclease-free water, 1 µL each of 0.5 µM forward and reverse primers, 0.25 µM probe, SARS-CoV-2 N-gene-specific primers, and TaqMan Fast Virus 1-Step Master Mix (Life Technologies, CA, USA) [16]. The SARS-CoV-2 primers and probes were described in a previous report [17]. The half-maximal inhibitory concentration, IC50 was calculated as follows: viral inhibition (%) = number of virus copies in [(virus control − sample) / virus control] × 100.
Virus titration by focus assay
The virus titer was determined using a focus-forming assay in 96-well plates containing Vero E6 cells as described previously, with some modifications [18]. Tenfold serial dilutions of infected cell supernatants were added to a 96-well plate in duplicate. After 1.5 h, 1.25% methylcellulose 4000 (Wako, Osaka, Japan) in 2% FCS MEM was added, and the plates were incubated at 37 °C and 5% CO2. Immunostaining was performed using a mouse monoclonal antibody against the SARS-CoV-2 N protein (ADTEC, Oita, Japan) as the primary antibody, followed by horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin G (IgG)-106 PU (American Qualex, CA, USA) as the secondary antibody. The bound conjugate was visualized by adding 3,3’-diaminobenzidine tetrahydrochloride (Wako, Tokyo, Japan) and hydrogen peroxide. Foci were counted, and the virus titer was expressed as focus-forming units per milliliter (FFU/mL).
Cell viability assay
To determine the concentration of inhibitor that caused a 50% reduction in cell survival (CC50), a cell viability assay was performed in parallel with the antiviral assay, using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) according to the manufacturer’s instructions (Promega, WI, USA). The optical density was measured at 570 nm using a microplate reader (Synergy H1 M, Biotech). Cell viability was calculated as follows: cell viability (%) = [(sample OD570)/(cell control OD570) × 100.
Immunofluorescence assay (IFA)
Vero E6 cells on 8-well glass slides (Millipore, Massachusetts, USA) were pre-treated with the teas or compounds for 1 h prior to viral infection and infected with SARS-CoV-2 at an MOI of 0.02 for 2 h. The inoculum was then removed, and the cells were washed with PBS. Tea or compound was then added, and after 2 days, the cells were fixed overnight with 4% paraformaldehyde (Wako, Osaka, Japan), permeabilized with 1% NP-40, and blocked using BlockAce (Yukijirushi, Sapporo, Japan) at room temperature. The cells were then stained with a mouse antibody against the SARS-CoV-2 N protein (Gene Tex, CA, USA) as the primary antibody and Alexa Fluor 488–conjugated goat anti-mouse IgG (Invitrogen, MA, USA) as the secondary antibody. Nuclei were stained with Hoechst 33342 dye (Southern Biotech, AL, USA), and the cells were imaged using a Keyence (BZ-X710) fluorescence microscope (Keyence, IL, USA) with a 10× lens.
Western blot (WB) analysis
Vero E6 cells were infected at an MOI of 0.1, harvested 16 h p.i., and lysed in RIPA buffer (Thermo Scientific, Illinois, USA). Equal volumes of cell lysate and 2× sample buffer (4% sodium dodecyl sulfate [SDS], 0.25 M Tris-HCl [pH 6.8], 2% 2-mercaptoethanol, 20% glycerol, and 0.01% bromophenol blue) were mixed and boiled at 100 °C for 10 min. The proteins in the sample were separated by 5%-20% SDS-polyacrylamide gel electrophoresis and blotted onto a polyvinylidene fluoride membrane. After blocking with 5% skim milk, the membrane was stained with a rabbit antibody against the SARS-CoV-2 N protein (Gene Tex, CA, USA; 1:500 dilution) as the primary antibody, followed by horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (102 PU, American Qualex, CA, USA). The protein bands were detected by immersing the membrane in 3,3′-diaminobenzidine tetrahydrochloride solution. For molecular weight estimation on the WB, Precision Plus ProteinTM Dual Color Standards (Bio-Rad, California, USA) were used. ImageJ software was used to measure the intensity of protein bands.
3CL protease (3CL pro) inhibition assay
Inhibition of SARS-CoV-2 3CL pro (main protease, M pro) by the GTBs, ECG, and EGCG was measured using a fluorogenic 3CL Protease, MBP-tagged (SARS-CoV-2) Assay Kit (BPS Bioscience, CA, USA). Briefly, 150 ng of recombinant SARS-CoV-2 3CL was incubated with different concentrations of GTB, EGCG, and ECG at room temperature for 30 min. The protease inhibitor GC376 was used as a positive control, and MEM was used as a negative control. After adding substrate, the reaction mixture was incubated for 4 h at room temperature. Fluorescence intensity was measured using a Synergy H1TM Hybrid Multi-Mode Microplate Reader (Bio Tek Instruments, Inc., VT, USA) at 360 nm excitation and 460 nm emission wavelength.
Statistical analysis
Data were analyzed using SPSS for Windows (version 16.0; IBM Corp., Armonk, NY) and GraphPad Prism 9 (GraphPad Software, Inc., San Diego, CA, USA). Continuous variables are presented as mean values ± standard deviation. Group comparisons were performed using one-way analysis of variance. Student’s t-test was used to compare continuous variables between groups. For all calculations, a P-value less than 0.05 was considered statistically significant.
Results
Inhibition of SARS-CoV-2 by green tea beverage and tea compounds measured by qRT-PCR
To evaluate the antiviral effect of the teas against SARS-CoV-2 infection, a qRT-PCR assay was carried out with different dilutions of GTB-A, GTB-B, GTB-C, GTB-D, GTB-E, and GTB-F. The PET-bottled green tea and compounds were diluted in MEM with 2% FCS at different dilutions and concentrations.
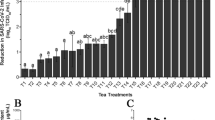
The antiviral remdesivir was used as a positive control (Fig. 1b), and the results showed that our assay was capable of measuring antiviral activity. As shown in Figure 1a, the six GTBs exhibited potent antiviral activity against SARS-CoV-2. The IC50 values for GTB (A–F) were 323-, 226-, 121-, 280-, 200-, and 192-fold dilutions (Fig. 1a). There was no cytotoxic effect in Vero E6 cells, and the CC50 values of the GTBs were < 25-fold dilution. To determine the virucidal activity of the natural polyphenol pigment compounds EGCG and ECG, the inhibitor treatment and infection procedures were performed as in the GTB experiments. The IC50 value for EGCG and ECG was 6.5 and 12.5 µM, respectively (Fig. 1b). The CC50 values for these two compounds were all >1000 µM.
Antiviral activity of GTB-A to -F (a) the tea compounds EGCG and ECG, and remdesivir (b) against SARS-CoV-2 at 2 days p.i. (MOI 0.02) and their cytotoxicity in Vero E6 cells. The blue and red lines represent the CC50 and IC50, respectively; the blue circles represent cell viability (%), and the red squares represent % inhibition of SARS-CoV-2 infection. The values are the mean ± SD (N = 3).
Inhibitory effect of green tea beverage and tea compounds measured by virus titration assay
We measured virus titers using a focus assay and confirmed that the GTBs, EGCG, and ECG inhibited the virus. Compared with the virus control, treatment with GTB-A-F (200-fold dilution) decreased inhibited the virus titer (p = 0.01–0.04) in Table 1. Additionally, treatment with 25 µM EGCG and ECG (p = 0.01) resulted in significantly reduced virus titers compared to virus control (Table 1).
Confirmation of the antiviral effect by IFA
To corroborate the viral inhibition results, we randomly selected three green teas (GTB-B, GTB-C, and GTB-E), as well as EGCG and ECG, and investigated their antiviral effect using an IFA. The viral N protein expression levels are shown in Fig. 2a and b according to tea dilution (25-fold, 50-fold dilution) and compound concentration (50 µM, 25 µM). A SARS-CoV-2 MOI of 0.02 was used in the assay. The virus was highly suppressed by treatment with 25- and 50-fold dilutions of GTB-B, GTB-C, and GTB-E and by treatment with 50 µM EGCG and ECG.
Immunofluorescence assay of SARS-CoV-2 (MOI 0.02) infection of Vero E6 cells treated with GTB-A to -F (a) and the tea compounds EGCG and ECG (b) at 2 days p.i. The confocal microscope images show cell nuclei (blue) and the SARS-CoV-2 nucleocapsid protein N (green). The mean ± SD was calculated from triplicate experiments.
Inhibition of virus entry measured by WB and qRT-PCR
For WB analysis, we randomly selected three GTBs (GTB-B, GTB-C, and GTB-E) at 50-fold dilution and EGCG and ECG at 50 µM, using DMSO as a control. The WB results showed that changes in NP expression levels in infected cells resulted in protein bands of different intensities (Fig. 3a and b) when inhibitors were present throughout the experiment (full treatment) or only during the post-entry or entry stage. Relative band intensities after normalizing to DMSO are shown in Fig. 3c and d). All substances exhibited effective inhibition when present throughout the experiment. Addition of inhibition at the post-entry and entry stages resulted in less inhibition.
Time-of-addition assay. SARS-CoV-2 viral protein was analyzed after addition of 50-fold dilutions of GTB-B, GTB-C, and GTB-E (a), 50 µM EGCG or ECG (b), or DMSO at different time points. N protein expression in cell lysates was analyzed by western blot at 16 h p.i. Band intensities were quantitated using ImageJ and are graphically displayed for GTB-B, GTB-C, and GTB-E (c), EGCG and ECG (d), and DMSO. The relative intensities of c and d were normalized to the DMSO control, which was set to 1.0.
The qRT-PCR quantification showed the highest level of inhibition when the inhibitor was constantly present followed by the post-entry and entry treatments with GTB-B, GTB-C, GTB-E, EGCG, and ECG (Fig. 4a and b). After normalizing to the virus control, the respective viral inhibition rates for the continuous (full treatment), post-entry, and entry treatments were as follows: GTB-B (99%, 94%, 88%), GTB-C (99%, 86%, 62%), GTB-E (97%, 90%, 63%), EGCG (73%, 77%, 52%), ECG (42%, 17%, 4%). The proportions of viral inhibition were significantly different among the continuous, post-entry, and entry stages for the GTBs and EGCG.
Time-of-addition assay for quantification of SARS-CoV-2 inhibition (%) after addition of 50-fold dilutions of GTB-B, GTB-C, and GTB-E (a) and 50 µM EGCG and ECG (b) at different time points. Viral RNA in cell supernatants was measured by qRT-PCR at 16 h p.i. The mean ± SD was calculated from triplicate experiments. *, p < 0.05; **, p < 0.01; **, p < 0.001. Student’s t-test was used to compare the different treatments after normalization to the virus control.
Inhibitory effect of GTB and tea compounds measured by 3CL protease assay
To investigate the reason for the higher virus inhibition rate in post-entry treatment than entry treatment, we tested the inhibition of SARS-CoV-2 Mpro by randomly selected GTBs (GTB-B, GTB-C, and GTB-E) and tea compounds, using a 3CL protease activity kit. The data showed that GTB-B, GTB-C, and GTB-E inhibited 3CLpro activity with IC50 values of 10-, 15- and 18-fold dilution, respectively. The EGCG and ECG tea compounds inhibited the 3CLpro activity with IC50 values of 56 µM and 110 µM, respectively.
Discussion
Previous reports have indicated that some green tea derivatives can inactivate SARS-CoV-2 [19, 20]. Our study reveals for the first time that a variety of PET-bottled Japanese green tea beverages inhibit SARS-CoV-2 infection in a dilution-dependent manner. Similar to the results of other studies, we also found that polyphenolic catechin compounds present in green tea (ECG and EGCG) had virucidal activity. Tea is a popular low-cost beverage worldwide. Many studies have shown it to have various biological effects, including antioxidant, anti-inflammatory, lipid metabolism-regulating, and anti-cancer effects [10, 19,20,21]. Tea has also been shown to suppress viral growth of members of the families Orthomyxoviridae, Picornaviridae, Flaviviridae, Retroviridae, Hepadnaviridae, Herpesviridae, Adenoviridae, and Phenuiviridae [22,23,24,25]. A 100-fold dilution of six types of GTBs inhibited SARS-CoV-2 by 70%–88% in terms of both the viral RNA level and infection titer. Depending on the cultivar, agricultural procedures, tea planting season, and levels of enzymatic oxidation during tea leaf processing, the catechin concentrations in tea may differ [19]. In this study, the investigated GTBs at 50-fold dilution potently suppressed SARS-CoV-2 infection in vitro, as observed by IFA.
Viral inhibition was observed to occur at the post-entry and entry stages of SARS-CoV-2 infection, as shown by WB and qRT-PCR. Catechins are metabolized by intestinal microbiota and are poorly absorbed by the gut after consumption; however, low levels of these compounds can be transported to the blood via the gastrointestinal tract [26, 27]. Commercial green tea typically contains 50–100 mg of catechins. It has been reported that the plasma concentration of EGCG was 0.6 µM when healthy volunteers consumed 150 mg of EGCG two times per day for five days [26, 28]. Drinking or gargling tea can inactivate SARS-CoV-2 in the oral cavity and pharynx, which can prevent viral entry. Thus, drinking or gargling tea or tea compounds might reduce person-to-person viral transmission because these substances might inactivate virions in the saliva of infected people [29]. As vitamin C is also included in PET-bottled GTB, we also tested the antiviral effect of vitamin C, pure ascorbic acid powder (Fujifilm, Osaka, Japan) against SARS-CoV-2 infection. The IC50 and CC50 of vitamin C was found to be 22.5 µM and >100 µM (Supplementary Fig. S1) using the same antiviral qRT-PCR assay used for the green teas. This suggests that not only catechins but also vitamin C has an antiviral effect on SARS-CoV-2 infection. As vitamin C concentrations may differ in PET-bottled GTBs, 70%-88% of SARS-CoV-2 virus inhibition was found in 100-fold dilution of GTBs. The authors of previous studies have also proposed using vitamin C to COVID-19 patients [30,31,32].
This demonstrates that compounds present in green tea (EGCG and ECG) inhibit SARS-CoV-2 in a dose-dependent manner. EGCG showed higher SARS-CoV-2 inactivation than ECG, as evidenced by the viral RNA level and infection titer. Notably, these results are consistent with those of previous in vitro studies on the inactivation of SARS-CoV-2 by catechins [19, 33,34,35,36]. Tea polyphenols can act on viral and cellular proteins during SARS-CoV-2 infection [37,38,39]. Catechins not only act on virus particles but also prevent fusion with target cells by interfering with endosome acidification and viral enzymes [23]. Computer modeling has suggested that EGCG might prevent SARS-CoV-2 infection by interacting with the cellular receptor ACE2 [38]. Consistent with this, we observed strong viral inhibition when this compound was applied during the post-entry stage, while the rate of inhibition was lower at the entry stage for GTBs, EGCG, and ECG. To investigate the possible mechanism of virus inhibition at the post-entry stage, we measured inhibition of SARS-CoV-2 3CL protease activity by GTBs, EGCG, and ECG and found an inhibitory effect of GTBs on the 3CL protease of SARS-CoV-2. In agreement with other studies [34, 36, 40,41,42], we observed inhibition of 3CL protease by EGCG and ECG. Consistent with earlier studies showing inhibition of SARS-CoV-2 entry by EGCG [43, 44], we observed inhibition at the entry stage of the viral life cycle.
Tea beverages have been the most widely consumed beverage for many centuries in Japan and other Asian countries, which have a lower mortality rate and incidence of SARS-CoV-2 infection than in Europe and the Americas, and this may have influenced the incidence of SARS-CoV-2 infection in this region [43, 45]. In Europe and the Americas, black tea is the dominant type of tea consumed. Theaflavin in black tea is poorly absorbed by the body, and its bioavailability is much lower than that of green tea, and this might have an effect on SARS-CoV-2 infection rates in these areas of the world [42, 46, 47]. An earlier study suggested that higher intake of GTB in Japan than in United States might partially explain the difference in mortality rates between two countries [48]. However, further detailed studies are needed to evaluate the effect of tea consumption on the spread of SARS-CoV-2 infection, and experimental epidemiological studies using GTBs and tea compounds to treat COVID-19 need to be performed. In conclusion, our study suggests that GTBs and tea compounds are potential candidates for prophylaxis and treatment of COVID-19.
References
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506
Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 323:1239–1242
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:271-280.e278
Ganesh B, Rajakumar T, Malathi M, Manikandan N, Nagaraj J, Santhakumar A, Elangovan A, Malik YS (2021) Epidemiology and pathobiology of SARS-CoV-2 (COVID-19) in comparison with SARS, MERS: an updated overview of current knowledge and future perspectives. Clin Epidemiol Glob Health 10:100694
Organization WH (2021) World Health Organization. Coronavirus disease (COVID-19) Pandemic. https://covid19.who.int/
Zahradník J, Marciano S, Shemesh M, Zoler E, Harari D, Chiaravalli J, Meyer B, Rudich Y, Li C, Marton I, Dym O, Elad N, Lewis MG, Andersen H, Gagne M, Seder RA, Douek DC, Schreiber G (2021) SARS-CoV-2 variant prediction and antiviral drug design are enabled by RBD in vitro evolution. Nat Microbiol 6:1188–1198
Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C (2020) Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395:1569–1578
Holman W, Holman W, McIntosh S, Painter W, Painter G, Bush J, Cohen O (2021) Accelerated first-in-human clinical trial of EIDD-2801/MK-4482 (molnupiravir), a ribonucleoside analog with potent antiviral activity against SARS-CoV-2. Trials 22:561
Rahman MM, Mosaddik A, Alam AK (2021) Traditional foods with their constituent’s antiviral and immune system modulating properties. Heliyon 7:e05957
Ohishi T, Goto S, Monira P, Isemura M, Nakamura Y (2016) Anti-inflammatory Action of green tea. Antiinflamm Antiallergy Agents Med Chem 15:74–90
Bellavite P, Donzelli A (2020) Hesperidin and SARS-CoV-2: new light on the healthy function of citrus fruits. Antioxidants (Basel) 9:742
Frank B, Conzelmann C, Weil T, Groß R, Jungke P, Eggers M, Müller JA, Münch J, Kessler U (2020) Antiviral activity of plant juices and green tea against SARS-CoV-2 and influenza virus in vitro. bioRxiv:2020.2010.2030.360545
Kwon PS, Oh H, Kwon SJ, Jin W, Zhang F, Fraser K, Hong JJ, Linhardt RJ, Dordick JS (2020) Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discov 6:50
Yim SK, Kim K, Kim IH, Chun SH, Oh TH, Kim JU, Kim JW, Jung WH, Moon HS, Ku BS, Jung KJ (2021) Inhibition of SARS-CoV-2 virus entry by the crude polysaccharides of seaweeds and abalone viscera in vitro. Mar Drugs 19:219
Khan N, Mukhtar H (2018) Tea polyphenols in promotion of human health. Nutrients 11:39
Ngwe Tun MM, Kyaw AK, Nabeshima T, Soe AM, Nwe KM, Htet KKK, Aung TH, Htwe TT, Aung T, Myaing SS, Mar TT, Lwin EP, Thu HM, Thant KZ, Morita K (2021) Detection of genotype-1 of dengue virus serotype 3 for the first time and complete genome analysis of dengue viruses during the 2018 epidemic in Mandalay, Upper Myanmar. PLoS ONE 16:e0251314
Shirato K, Nao N, Katano H, Takayama I, Saito S, Kato F, Katoh H, Sakata M, Nakatsu Y, Mori Y, Kageyama T, Matsuyama S, Takeda M (2020) Development of genetic diagnostic methods for detection for novel coronavirus 2019 (nCoV-2019) in Japan. Jpn J Infect Dis 73:304–307
Ngwe Tun MM, Thant KZ, Inoue S, Kurosawa Y, Lwin YY, Lin S, Aye KT, Thet Khin P, Myint T, Htwe K, Mapua CA, Natividad FF, Hirayama K, Morita K (2013) Serological characterization of dengue virus infections observed among dengue hemorrhagic fever/dengue shock syndrome cases in upper Myanmar. J Med Virol 85:1258–1266
Ohgitani E, Shin-Ya M, Ichitani M, Kobayashi M, Takihara T, Kawamoto M, Kinugasa H, Mazda O (2021) Significant inactivation of SARS-CoV-2 in vitro by a green tea catechin, a catechin-derivative, and black tea galloylated theaflavins. Molecules (Basel, Switzerland) 26:3572
Hisanaga A, Ishida H, Sakao K, Sogo T, Kumamoto T, Hashimoto F, Hou DX (2014) Anti-inflammatory activity and molecular mechanism of Oolong tea theasinensin. Food Funct 5:1891–1897
Xu J, Xu Z, Zheng W (2017) A review of the antiviral role of green tea catechins. Molecules (Basel, Switzerland) 22:1337
Nishimura H, Okamoto M, Dapat I, Katsumi M, Oshitani H (2021) Inactivation of SARS-CoV-2 by catechins from green tea. Jpn J Infect Dis 74:421–423
Steinmann J, Buer J, Pietschmann T, Steinmann E (2013) Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br J Pharmacol 168:1059–1073
Ogawa M, Shimojima M, Saijo M, Fukasawa M (2021) Several catechins and flavonols from green tea inhibit severe fever with thrombocytopenia syndrome virus infection in vitro. J Infect Chemother 27:32–39
Raekiansyah M, Buerano CC, Luz MAD, Morita K (2018) Inhibitory effect of the green tea molecule EGCG against dengue virus infection. Arch Virol 163:1649–1655
Scholl C, Lepper A, Lehr T, Hanke N, Schneider KL, Brockmöller J, Seufferlein T, Stingl JC (2018) Population nutrikinetics of green tea extract. PLoS ONE 13:e0193074
Cai ZY, Li XM, Liang JP, Xiang LP, Wang KR, Shi YL, Yang R, Shi M, Ye JH, Lu JL, Zheng XQ, Liang YR (2018) Bioavailability of tea catechins and its improvement. Molecules (Basel, Switzerland) 23:2346
Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS (2002) Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomark Prev 11:1025–1032
Ohgitani E, Shin-Ya M, Ichitani M, Kobayashi M, Takihara T, Kawamoto M, Kinugasa H, Mazda O (2021) Rapid inactivation in vitro of SARS-CoV-2 in saliva by black tea and green tea. Pathogens 10:721
Carr AC, Rowe S (2020) The emerging role of vitamin C in the prevention and treatment of COVID-19. Nutrients 12:3286
Hemilä H, de Man AME (2021) Vitamin C and COVID-19. Front Med. https://doi.org/10.3389/fmed.2020.559811
Al Sulaiman K, Aljuhani O, Saleh KB, Badreldin HA, Al Harthi A, Alenazi M, Alharbi A, Algarni R, Al Harbi S, Alhammad AM, Vishwakarma R, Aldekhyl S (2021) Author Correction: Ascorbic acid as an adjunctive therapy in critically ill patients with COVID-19: a propensity score matched study. Sci Rep 11:19433
Jang M, Park YI, Cha YE, Park R, Namkoong S, Lee JI, Park J (2020) Tea polyphenols EGCG and theaflavin inhibit the activity of SARS-CoV-2 3CL-protease in vitro. Evid Based Complement Altern Med 2020:5630838
Bahun M, Jukić M, Oblak D, Kranjc L, Bajc G, Butala M, Bozovičar K, Bratkovič T, Podlipnik Č, Poklar Ulrih N (2022) Inhibition of the SARS-CoV-2 3CL(pro) main protease by plant polyphenols. Food Chem 373:131594
Du A, Zheng R, Disoma C, Li S, Chen Z, Li S, Liu P, Zhou Y, Shen Y, Liu S, Zhang Y, Dong Z, Yang Q, Alsaadawe M, Razzaq A, Peng Y, Chen X, Hu L, Peng J, Zhang Q, Jiang T, Mo L, Li S, Xia Z (2021) Epigallocatechin-3-gallate, an active ingredient of traditional Chinese medicines, inhibits the 3CLpro activity of SARS-CoV-2. Int J Biol Macromol 176:1–12
Zhu Y, Xie DY (2020) Docking characterization and in vitro inhibitory activity of flavan-3-ols and dimeric proanthocyanidins against the main protease activity of SARS-Cov-2. Front Plant Sci 11:601316
Hensel A, Bauer R, Heinrich M, Spiegler V, Kayser O, Hempel G, Kraft K (2020) Challenges at the time of COVID-19: opportunities and innovations in antivirals from nature. Planta Med 86:659–664
Mhatre S, Srivastava T, Naik S, Patravale V (2021) Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: a review. Phytomedicine 85:153286
Chourasia M, Koppula PR, Battu A, Ouseph MM, Singh AK (2021) EGCG, a Green tea catechin, as a potential therapeutic agent for symptomatic and asymptomatic SARS-CoV-2 infection. Molecules (Basel, Switzerland) 26:1200
Kato Y, Higashiyama A, Takaoka E, Nishikawa M, Ikushiro S (2021) Food phytochemicals, epigallocatechin gallate and myricetin, covalently bind to the active site of the coronavirus main protease in vitro. Adv Redox Res 3:100021
Boras B, Jones RM, Anson BJ, Arenson D, Aschenbrenner L, Bakowski MA, Beutler N, Binder J, Chen E, Eng H, Hammond H, Hammond J, Haupt RE, Hoffman R, Kadar EP, Kania R, Kimoto E, Kirkpatrick MG, Lanyon L, Lendy EK, Lillis JR, Logue J, Luthra SA, Ma C, Mason SW, McGrath ME, Noell S, Obach RS, O’Brien MN, O’Connor R, Ogilvie K, Owen D, Pettersson M, Reese MR, Rogers TF, Rosales R, Rossulek MI, Sathish JG, Shirai N, Steppan C, Ticehurst M, Updyke LW, Weston S, Zhu Y, White KM, García-Sastre A, Wang J, Chatterjee AK, Mesecar AD, Frieman MB, Anderson AS, Allerton C (2021) Preclinical characterization of an intravenous coronavirus 3CL protease inhibitor for the potential treatment of COVID19. Nat Commun 12:6055
Park J, Park R, Jang M, Park YI (2021) Therapeutic potential of EGCG, a green tea polyphenol, for treatment of coronavirus diseases. Life (Basel) 11:197
Liu J, Bodnar BH, Meng F, Khan AI, Wang X, Saribas S, Wang T, Lohani SC, Wang P, Wei Z, Luo J, Zhou L, Wu J, Luo G, Li Q, Hu W, Ho W (2021) Epigallocatechin gallate from green tea effectively blocks infection of SARS-CoV-2 and new variants by inhibiting spike binding to ACE2 receptor. Cell Biosci 11:168
Henss L, Auste A, Schürmann C, Schmidt C, von Rhein C, Mühlebach MD, Schnierle BS (2021) The green tea catechin epigallocatechin gallate inhibits SARS-CoV-2 infection. J Gen Virol 102:001574
Storozhuk M (2021) COVID -19: could green tea catechins reduce the risks? medRxiv:2020.2010.2023.20218479
Mulder TP, van Platerink CJ, Wijnand Schuyl PJ, van Amelsvoort JM (2001) Analysis of theaflavins in biological fluids using liquid chromatography-electrospray mass spectrometry. J Chromatogr B Biomed Sci Appl 760:271–279
Pereira-Caro G, Moreno-Rojas JM, Brindani N, Del Rio D, Lean MEJ, Hara Y, Crozier A (2017) Bioavailability of black tea theaflavins: absorption, metabolism, and colonic catabolism. J Agric Food Chem 65:5365–5374
Kagawa Y (2022) Influence of nutritional intakes in Japan and the United States on COVID-19 infection. Nutrients 14:633
Acknowledgments
We thank all the members of the Department of Virology, Institute of Tropical Medicine, Nagasaki University, Japan. This research was performed by using the INM deposited WAKANYAKU library, Institute of Natural Medicine, University of Toyama, Japan.
Funding
This research was supported by the Japan Agency for Medical Research and Development (AMED) under grant number JP22wm0125006 (Japan Program for Infectious Diseases Research and Infrastructure).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling Editor: William G Dundon .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ngwe Tun, M.M., Luvai, E., Nwe, K.M. et al. Anti-SARS-CoV-2 activity of various PET-bottled Japanese green teas and tea compounds in vitro. Arch Virol 167, 1547–1557 (2022). https://doi.org/10.1007/s00705-022-05483-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-022-05483-x