Abstract
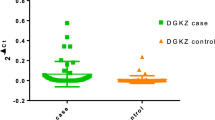
Earlier studies have implicated CHRNA7, coding α-7 nicotinic acetylcholine receptor (α7 nAChR), and its partially duplicated chimeric gene CHRFAM7A in schizophrenia. However, the relationship between the alterations in peripheral gene expression of CHRFAM7A and severity of clinical symptoms has not been examined. Furthermore, potential influence of the antipsychotic medication on CHRFAM7A expression in drug-naive or drug-free schizophrenia is an unexplored area. CHRFAM7A gene expression in lymphocytes was analyzed in 90 antipsychotic-naïve or free schizophrenia patients using TaqMan-based quantitative RT-PCR. Psychotic symptoms were assessed using Scale for Assessment of Positive Symptoms and Scale for Assessment of Negative Symptoms (SANS). The relationship between psychopathology and CHRFAM7A expression was examined. In addition, measurement of CHRFAM7A gene expression was repeated during follow-up after short-term antipsychotic treatment in 38 patients. There was significant inverse correlation between CHRFAM7A expression and total negative psychopathology score—SANS, and this relationship persisted after accounting for possible confounders such as age, sex and smoking. On exploration of the factor structure of psychopathology using principal component analysis, all the negative symptoms—affective flattening, alogia, apathy, anhedonia and inattention were found to be inversely associated with CHRFAM7A expression. Furthermore, analysis of repeated measures revealed a significant increase in CHRFAM7A expression in patients after short-term administration of antipsychotic medication. Our study observations support the role for CHRFAM7A gene in schizophrenia pathogenesis and suggest a potential novel link between deficient CHRFAM7A expression and negative psychopathology. Furthermore, up-regulation of CHRFAM7A gene expression by antipsychotics suggests that it could be a potential state marker for clinical severity.



Similar content being viewed by others
References
Bencherif M, Stachowiak MK, Kucinski AJ, Lippiello PM (2012) Alpha7 nicotinic cholinergic neuromodulation may reconcile multiple neurotransmitter hypotheses of schizophrenia. Med Hypotheses 78(5):594–600. https://doi.org/10.1016/j.mehy.2012.01.035
Bertelsen B, Oranje B, Melchior L, Fagerlund B, Werge TM, Mikkelsen JD, Tumer Z, Glenthoj BY (2015) Association study of CHRNA7 promoter variants with sensory and sensorimotor gating in schizophrenia patients and healthy controls: a Danish case-control study. NeuroMol Med 17(4):423–430. https://doi.org/10.1007/s12017-015-8371-9
de Lucas-Cerrillo AM, Maldifassi MC, Arnalich F, Renart J, Atienza G, Serantes R, Cruces J, Sanchez-Pacheco A, Andres-Mateos E, Montiel C (2011) Function of partially duplicated human alpha77 nicotinic receptor subunit CHRFAM7A gene: potential implications for the cholinergic anti-inflammatory response. J Biol Chem 286(1):594–606. https://doi.org/10.1074/jbc.M110.180067
D’Souza DC, Esterlis I, Carbuto M, Krasenics M, Seibyl J, Bois F, Pittman B, Ranganathan M, Cosgrove K, Staley J (2012) Lower ss2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia. Am J Psychiatry 169(3):326–334. https://doi.org/10.1176/appi.ajp.2011.11020189
Esterlis I, Ranganathan M, Bois F, Pittman B, Picciotto MR, Shearer L, Anticevic A, Carlson J, Niciu MJ, Cosgrove KP, D’Souza DC (2014) In vivo evidence for beta2 nicotinic acetylcholine receptor subunit upregulation in smokers as compared with nonsmokers with schizophrenia. Biol Psychiatry 76(6):495–502. https://doi.org/10.1016/j.biopsych.2013.11.001
Freedman R, Adams CE, Leonard S (2000) The alpha7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. J Chem Neuroanat 20(3–4):299–306
Hong LE, Yang X, Wonodi I, Hodgkinson CA, Goldman D, Stine OC, Stein ES, Thaker GK (2011) A CHRNA5 allele related to nicotine addiction and schizophrenia. Genes Brain Behav 10(5):530–535. https://doi.org/10.1111/j.1601-183X.2011.00689.x
Husson F, Josse J, Le S, Mazet J (2014) FactoMineR: multivariate exploratory data analysis and data mining with R. 1.27 edn
Jackson KJ, Fanous AH, Chen J, Kendler KS, Chen X (2013) Variants in the 15q25 gene cluster are associated with risk for schizophrenia and bipolar disorder. Psychiatr Genet 23(1):20–28. https://doi.org/10.1097/YPG.0b013e32835bd5f1
John JP, Khanna S, Thennarasu K, Reddy S (2003) Exploration of dimensions of psychopathology in neuroleptic-naive patients with recent-onset schizophrenia/schizophreniform disorder. Psychiatry Res 121(1):11–20
Koukouli F, Rooy M, Tziotis D, Sailor KA, O’Neill HC, Levenga J, Witte M, Nilges M, Changeux JP, Hoeffer CA, Stitzel JA, Gutkin BS, DiGregorio DA, Maskos U (2017) Nicotine reverses hypofrontality in animal models of addiction and schizophrenia. Nat Med 23(3):347–354. https://doi.org/10.1038/nm.4274
Kunii Y, Zhang W, Xu Q, Hyde TM, McFadden W, Shin JH, Deep-Soboslay A, Ye T, Li C, Kleinman JE, Wang KH, Lipska BK (2015) CHRNA7 and CHRFAM7A mRNAs: co-localized and their expression levels altered in the postmortem dorsolateral prefrontal cortex in major psychiatric disorders. Am J Psychiatry 172(11):1122–1130. https://doi.org/10.1176/appi.ajp.2015.14080978
Le S, Josse J, Husson F (2008) FactoMineR: An R Package for Multivariate Analysis. J Stat Softw 25(1):1–18. https://doi.org/10.18637/jss.v025.i01
Leonard S, Freedman R (2006) Genetics of chromosome 15q13–q14 in schizophrenia. Biol Psychiatry 60(2):115–122. https://doi.org/10.1016/j.biopsych.2006.03.054
Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, Williams NM, Schwab SG, Pulver AE, Faraone SV, Brzustowicz LM, Kaufmann CA, Garver DL, Gurling HM, Lindholm E, Coon H, Moises HW, Byerley W, Shaw SH, Mesen A, Sherrington R, O’Neill FA, Walsh D, Kendler KS, Ekelund J, Paunio T, Lonnqvist J, Peltonen L, O’Donovan MC, Owen MJ, Wildenauer DB, Maier W, Nestadt G, Blouin JL, Antonarakis SE, Mowry BJ, Silverman JM, Crowe RR, Cloninger CR, Tsuang MT, Malaspina D, Harkavy-Friedman JM, Svrakic DM, Bassett AS, Holcomb J, Kalsi G, McQuillin A, Brynjolfson J, Sigmundsson T, Petursson H, Jazin E, Zoega T, Helgason T (2003) Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: schizophrenia. Am J Hum Genet 73(1):34–48. https://doi.org/10.1086/376549
Li J (2008) A two-step rejection procedure for testing multiple hypotheses. J Stat Plan Inference 138(6):1521–1527
Mackowick KM, Barr MS, Wing VC, Rabin RA, Ouellet-Plamondon C, George TP (2014) Neurocognitive endophenotypes in schizophrenia: modulation by nicotinic receptor systems. Prog Neuropsychopharmacol Biol Psychiatry 52:79–85. https://doi.org/10.1016/j.pnpbp.2013.07.010
Marshall CR, Howrigan DP, Merico D, Thiruvahindrapuram B, Wu W, Greer DS, Antaki D, Shetty A, Holmans PA, Pinto D, Gujral M, Brandler WM, Malhotra D, Wang Z, Fajarado KVF, Maile MS, Ripke S, Agartz I, Albus M, Alexander M, Amin F, Atkins J, Bacanu SA, Belliveau RA Jr, Bergen SE, Bertalan M, Bevilacqua E, Bigdeli TB, Black DW, Bruggeman R, Buccola NG, Buckner RL, Bulik-Sullivan B, Byerley W, Cahn W, Cai G, Cairns MJ, Campion D, Cantor RM, Carr VJ, Carrera N, Catts SV, Chambert KD, Cheng W, Cloninger CR, Cohen D, Cormican P, Craddock N, Crespo-Facorro B, Crowley JJ, Curtis D, Davidson M, Davis KL, Degenhardt F, Del Favero J, DeLisi LE, Dikeos D, Dinan T, Djurovic S, Donohoe G, Drapeau E, Duan J, Dudbridge F, Eichhammer P, Eriksson J, Escott-Price V, Essioux L, Fanous AH, Farh KH, Farrell MS, Frank J, Franke L, Freedman R, Freimer NB, Friedman JI, Forstner AJ, Fromer M, Genovese G, Georgieva L, Gershon ES, Giegling I, Giusti-Rodriguez P, Godard S, Goldstein JI, Gratten J, de Haan L, Hamshere ML, Hansen M, Hansen T, Haroutunian V, Hartmann AM, Henskens FA, Herms S, Hirschhorn JN, Hoffmann P, Hofman A, Huang H, Ikeda M, Joa I, Kahler AK, Kahn RS, Kalaydjieva L, Karjalainen J, Kavanagh D, Keller MC, Kelly BJ, Kennedy JL, Kim Y, Knowles JA, Konte B, Laurent C, Lee P, Lee SH, Legge SE, Lerer B, Levy DL, Liang KY, Lieberman J, Lonnqvist J, Loughland CM, Magnusson PKE, Maher BS, Maier W, Mallet J, Mattheisen M, Mattingsdal M, McCarley RW, McDonald C, McIntosh AM, Meier S, Meijer CJ, Melle I, Mesholam-Gately RI, Metspalu A, Michie PT, Milani L, Milanova V, Mokrab Y, Morris DW, Muller-Myhsok B, Murphy KC, Murray RM, Myin-Germeys I, Nenadic I, Nertney DA, Nestadt G, Nicodemus KK, Nisenbaum L, Nordin A, O’Callaghan E, O’Dushlaine C, Oh SY, Olincy A, Olsen L, O’Neill FA, Van Os J, Pantelis C, Papadimitriou GN, Parkhomenko E, Pato MT, Paunio T, Perkins DO, Pers TH, Pietilainen O, Pimm J, Pocklington AJ, Powell J, Price A, Pulver AE, Purcell SM, Quested D, Rasmussen HB, Reichenberg A, Reimers MA, Richards AL, Roffman JL, Roussos P, Ruderfer DM, Salomaa V, Sanders AR, Savitz A, Schall U, Schulze TG, Schwab SG, Scolnick EM, Scott RJ, Seidman LJ, Shi J, Silverman JM, Smoller JW, Soderman E, Spencer CCA, Stahl EA, Strengman E, Strohmaier J, Stroup TS, Suvisaari J, Svrakic DM, Szatkiewicz JP, Thirumalai S, Tooney PA, Veijola J, Visscher PM, Waddington J, Walsh D, Webb BT, Weiser M, Wildenauer DB, Williams NM, Williams S, Witt SH, Wolen AR, Wormley BK, Wray NR, Wu JQ, Zai CC, Adolfsson R, Andreassen OA, Blackwood DHR, Bramon E, Buxbaum JD, Cichon S, Collier DA, Corvin A, Daly MJ, Darvasi A, Domenici E, Esko T, Gejman PV, Gill M, Gurling H, Hultman CM, Iwata N, Jablensky AV, Jonsson EG, Kendler KS, Kirov G, Knight J, Levinson DF, Li QS, McCarroll SA, McQuillin A, Moran JL, Mowry BJ, Nothen MM, Ophoff RA, Owen MJ, Palotie A, Pato CN, Petryshen TL, Posthuma D, Rietschel M, Riley BP, Rujescu D, Sklar P, St Clair D, Walters JTR, Werge T, Sullivan PF, O’Donovan MC, Scherer SW, Neale BM, Sebat J (2017) Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat Genet 49(1):27–35. https://doi.org/10.1038/ng.3725
Martin LF, Freedman R (2007) Schizophrenia and the alpha7 nicotinic acetylcholine receptor. Int Rev Neurobiol 78:225–246. https://doi.org/10.1016/s0074-7742(06)78008-4
Ota VK, Noto C, Gadelha A, Santoro ML, Silva PN, Melaragno MI, Smith Mde A, Cordeiro Q, Bressan RA, Belangero SI (2013) Neurotransmitter receptor and regulatory gene expression in peripheral blood of Brazilian drug-naive first-episode psychosis patients before and after antipsychotic treatment. Psychiatry Res 210(3):1290–1292. https://doi.org/10.1016/j.psychres.2013.09.016
Parikh V, Kutlu MG, Gould TJ (2016) nAChR dysfunction as a common substrate for schizophrenia and comorbid nicotine addiction: current trends and perspectives. Schizophr Res 171(1–3):1–15. https://doi.org/10.1016/j.schres.2016.01.020
Peralta V, Cuesta MJ (1999) Dimensional structure of psychotic symptoms: an item-level analysis of SAPS and SANS symptoms in psychotic disorders. Schizophr Res 38(1):13–26
Perl O, Ilani T, Strous RD, Lapidus R, Fuchs S (2003) The alpha7 nicotinic acetylcholine receptor in schizophrenia: decreased mRNA levels in peripheral blood lymphocytes. FASEB J 17(13):1948–1950. https://doi.org/10.1096/fj.03-0104fje
Perl O, Strous RD, Dranikov A, Chen R, Fuchs S (2006) Low levels of alpha7-nicotinic acetylcholine receptor mRNA on peripheral blood lymphocytes in schizophrenia and its association with illness severity. Neuropsychobiology 53(2):88–93. https://doi.org/10.1159/000091725
Petrovsky N, Quednow BB, Ettinger U, Schmechtig A, Mossner R, Collier DA, Kuhn KU, Maier W, Wagner M, Kumari V (2010) Sensorimotor gating is associated with CHRNA3 polymorphisms in schizophrenia and healthy volunteers. Neuropsychopharmacology 35(7):1429–1439. https://doi.org/10.1038/npp.2010.12
Pinheiro J, Bates D, DebRoy S, Sarkar D, Team R-C (2015) {nlme}: linear and nonlinear mixed effects models. 3.1–120 edn. https://CRAN.R-project.org/package=nlm
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
RStudio Team (2013) RStudio: Integrated Development for R. RStudio, Inc., Boston, MA. http://www.rstudio.com/
Sato KZ, Fujii T, Watanabe Y, Yamada S, Ando T, Kazuko F, Kawashima K (1999) Diversity of mRNA expression for muscarinic acetylcholine receptor subtypes and neuronal nicotinic acetylcholine receptor subunits in human mononuclear leukocytes and leukemic cell lines. Neurosci Lett 266(1):17–20
Sequeira PA, Martin MV, Vawter MP (2012) The first decade and beyond of transcriptional profiling in schizophrenia. Neurobiol Dis 45(1):23–36. https://doi.org/10.1016/j.nbd.2011.03.001
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20):22–33 (quiz 34–57)
Sinkus ML, Graw S, Freedman R, Ross RG, Lester HA, Leonard S (2015) The human CHRNA7 and CHRFAM7A genes: a review of the genetics, regulation, and function. Neuropharmacology 96(Pt B):274–288. https://doi.org/10.1016/j.neuropharm.2015.02.006
Stephens SH, Logel J, Barton A, Franks A, Schultz J, Short M, Dickenson J, James B, Fingerlin TE, Wagner B, Hodgkinson C, Graw S, Ross RG, Freedman R, Leonard S (2009) Association of the 5′-upstream regulatory region of the alpha7 nicotinic acetylcholine receptor subunit gene (CHRNA7) with schizophrenia. Schizophr Res 109(1–3):102–112. https://doi.org/10.1016/j.schres.2008.12.017
Sullivan PF, Fan C, Perou CM (2006) Evaluating the comparability of gene expression in blood and brain. Am J Med Genet B Neuropsychiatr Genet 141B(3):261–268. https://doi.org/10.1002/ajmg.b.30272
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer, New York
Wickham H (2014) tidyr: Easily Tidy Data with spread() and gather() Functions. 0.2.0 edn. R package version 0.2.0. https://CRAN.R-project.org/package=tidyr
Wickham H, Francois R (2015) dplyr: A Grammar of Data Manipulation. R package version 0.7.4. https://CRAN.R-project.org/package=dplyr
Wium-Andersen MK, Orsted DD, Nordestgaard BG (2015) Tobacco smoking is causally associated with antipsychotic medication use and schizophrenia, but not with antidepressant medication use or depression. Int J Epidemiol 44(2):566–577. https://doi.org/10.1093/ije/dyv090
Zhou D, Gochman P, Broadnax DD, Rapoport JL, Ahn K (2016) 15q13.3 duplication in two patients with childhood-onset schizophrenia. Am J Med Genet B Neuropsychiatr Genet 171(6):777–783. https://doi.org/10.1002/ajmg.b.32439
Acknowledgements
This work is supported by the CEIB Programme Support Grant to GV (BT/PR5322/COE/34/8/2012) as well as by the Wellcome Trust/DBT India Alliance Senior Fellowship research grant to GV (500236/Z/11/Z). SVK, SMA and ACA are supported by the Wellcome Trust/DBT India Alliance. VS is supported by the Indian Council of Medical Research (DHR/HRD/Young Scientist/Type-VI-(2)/2015). AR is supported by the DST-INSPIRE (IF-120727). RA is supported by the CEIB Programme Support Grant. MS is supported by the UGC-RGNF & DV is supported by the UGC-NET-JRF. We sincerely acknowledge the reviewers inputs during the revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no potential conflicts of interest to report.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kalmady, S.V., Agrawal, R., Venugopal, D. et al. CHRFAM7A gene expression in schizophrenia: clinical correlates and the effect of antipsychotic treatment. J Neural Transm 125, 741–748 (2018). https://doi.org/10.1007/s00702-017-1833-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-017-1833-5