Abstract
Background
Cognitive dysfunction is the most common form of neurological impairment after aneurysmal subarachnoid hemorrhage (aSAH) in the chronic phase. Cognitive deficits in the acute phase after aSAH, however, remain scarcely investigated. The aim of the present study was to test cognitive function and to identify medical predictors of cognitive deficits in the acute phase of aSAH.
Methods
Prospective study including 51 patients treated for aSAH. Patients were treated in accordance with a standardized institutional protocol and subjected to neuropsychological evaluation around discharge from neurosurgical care. The neuropsychological test results were transformed into a global cognitive impairment index where an index value of 0.00 is considered normal and 1.00 is considered maximally pathological. Patients with an index score of less than 0.75 were considered having good global cognitive function while those with an index score equal to or above 0.75 were considered having poor global cognitive function. Univariate and multiple regression analysis were used to identify medical predictors of cognitive function.
Results
Fifty-seven percent of the patients had poor cognitive function. They showed severe cognitive deficits, with most tests falling well below two standard deviations from the expected normal mean. Poor cognitive function was not reflected in a poor modified Rankin score in almost half of the cases. Patients with good cognitive function showed only mild cognitive deficits with most tests falling only slightly below the normal mean. Delayed memory was the most affected function in both groups. Univariate analysis identified acute hydrocephalus and aSAH-acquired cerebral infarction to be predictors of poor cognitive function. Cerebrospinal fluid drainage in excess of 2000 ml six-folded the risk of poor cognitive function, whereas a new cerebral infarction 11-folded the respective risk of poor cognitive function.
Conclusion
More than half of aSAH patients have severe cognitive deficits in the acute phase. The modified Rankin Score should be combined with neuropsychological screening in the acute phase after aSAH to get a more accurate description of the patients’ disabilities. Acute hydrocephalus and aSAH-acquired cerebral infarction are the strongest predictors of poor cognitive function in the acute phase.
Similar content being viewed by others
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a potentially life-threatening disease which affects approximately 400 patients in Norway each year [23]. Among the survivors, as many as 65% experience changes in cognitive function. Cognitive dysfunction is hence the most common form of neurological impairment after aSAH [15]. Furthermore, it is well known that even patients recovering from an aSAH without physical deficits may function poorly due to mild to moderate cognitive deficits in the chronic phase [28]. Regarding cognitive deficits in the acute phase of aSAH, there is, however, limited research available. A study by Hütter and colleagues in 1998 [14] found impairments in short- and long-term memory, divided attention, and frontocortical functions in the acute phase (mean 5.9 days after aSAH). This pattern of cognitive deficits is quite similar to the cognitive deficits seen in the chronic phases after aSAH [26, 30, 31]. Moreover, most studies have based the predictive value of medical variables on cognitive dysfunction in the later or chronic phases after aSAH, often 3 months or 1 year after the ictus, so to the best of our knowledge, the study by Hütter [14] remains the sole study of aSAH patients in the acute phase that has related medical variables to cognitive function and they found that amount of blood (Fisher score), along with frontal hematoma, intraventricular hemorrhage, and acute hydrocephalus were related to cognitive dysfunction in the acute phase [14]. With continuing improvements in treating aSAH, these 20-year-old results should be validated, thereby hopefully contributing to isolate variables that can be modified in the management of aSAH so that cognitive deficits become minimized.
Further, in order to optimize rehabilitation efforts in aSAH patients, it is also essential to understand the medical predictors for cognitive malfunction. With that knowledge, patients in danger of more serious deficits could be identified readily and rehabilitation efforts could be initiated much earlier, possibly alleviating the cognitive consequences of the hemorrhage. Therefore, the aim of the present study was to describe the cognitive deficits seen in the acute phase after aSAH and to identify possible medical predictors of cognitive dysfunction.
Material and methods
Patients
Adult patients admitted to the Department of Neurosurgery, Oslo University Hospital, with aSAH that underwent aneurysm repair during 2012, were invited to participate. We excluded patients with previous aSAH or pre-existing cerebral neurodegenerative disorders and/or severe psychiatric illnesses. Patients that still were on invasive respiratory support at discharge or otherwise unable to perform neuropsychological investigations were also excluded. Patients that scored less than 70 points on the Galveston Orientation and Amnesia Test [22] were excluded. Patients were treated in accordance with our standardized institutional treatment guidelines for patients with aSAH [33]. When no longer in need of neurosurgical treatment, patients were discharged to their local hospital.
The present study is part of a larger project entitled: “Effect of early rehabilitation after aneurysmal SAH” and was approved by the Regional Committee for Medical Research Ethics, South-East Norway in January 2012, archive number 2011/2189. Clinical Trials number:0925-0586. Oral and written consent was obtained from all patients included.
Methods
Clinical data
Demographic data, social history, and medical history were obtained through medical records and interviews with patients and their families. Patients were scored according to the Hunt and Hess score [12], and Glasgow Coma Scale [18] just prior to aneurysm repair or prior to intubation. We registered if there was evidence for re-bleeding before securing of the aneurysm. Furthermore, we registered the mode of aneurysm repair, amount of cerebrospinal fluid (CSF) drainage, and occurrence of cerebral vasospasm. The following radiological variables were registered: (1) aneurysm site; (2) amount of SAH applying the modified Fisher score [8]; (3) amount of intraventricular blood according to Le Roux [21], where we assigned a “0” in subjects in whom there was no intraventricular blood present; (4) new, aSAH-acquired low attenuating lesions described as radiological signs of cerebral infarction; and (5) largest Evans index on any computed tomography (CT) scan during the primary stay (i.e., the ratio of maximum width of the frontal horns of the lateral ventricles and maximal internal diameter of the skull at the same level in axial CT/MRI) which is a useful marker of ventricular volume. Evans’ index greater than 0.3 was employed as a cut-off for increased ventricular size [35]. We also registered the modified Rankin Score [27] at discharge.
Neuropsychological testing
All patients included underwent standardized neuropsychological tests as close to the day of discharge as possible, see Table 1. Patients were tested while sitting in their bed or in a chair. Screens were drawn to minimize outside disturbances and to block out as much noise as possible. During the neuropsychological test, patients were left undisturbed by nursing care or other medical interventions. All tests were administered in the same order to all patients.
All neuropsychological tests were scored using published norms and converted to T-scores with a mean of 50 ± 10. This allows for comparison with a normal population with similar demographic features. We denoted T-scores between 45 and 40 (i.e., score between 0.5 and 1.0 SD below mean) as mild deficits, those between 40 and 30 (i.e., scores between 1.0 and 2.0 SD below mean) as moderate deficits, and T-scores less than 30 (i.e., larger than 2.0 SD below mean) as severe deficits. Further, a global cognitive impairment index (GCII) was calculated for each patient by dividing the number of significant parameters (test results with T-score below 40) with the total number of tests. An index value of 0.00 is considered normal and 1.00 is considered maximally pathological [25]. Patients with an index score of less than 0.75 were considered having good global cognitive function while those with an index score equal to or above 0.75 were considered having poor global cognitive function.
Statistical analysis
Descriptive data are shown by proportion mean values with SD or median with interquartile range (IQR). Explorative data analysis (Kolmogorov-Smirnov and Shapiro Wilk) demonstrated that the distribution of test results varied. Therefore, since the comparison had to cover both normally and non-normally distributed data, a non-parametric test (Mann-Whitney U test for continuous data and chi-square for categorical data) was applied. A logistic regression based on good/poor cognitive function was performed and the most significant predictors were further included in a step-wise backward multivariate regression. Results were considered significant when p < 0.05. Statistical analysis was conducted according to the procedures of IBM SPSS Statistics 25 for Windows.
Results
Demographic and clinical characteristics
A total of 140 patients with aSAH were admitted to our department in 2012. Nine patients did not undergo aneurysm repair, 34 were excluded (2 children, 18 patients were intubated at discharge, 8 died at the ICU, 5 had previous aSAH, and 1 had neurodegenerative disease). Among the remaining 97 patients, 51 were able to complete the neuropsychological test battery (Table 2), at a median 11 days after aSAH (range 2–45 days). Thirty of the patients were female, whereas 21 were male. Median age was 51 years (range 26–76 years). The patients included had a median of 12 years of education (range 8–20). Ninety-two percent of the included patients were in Hunt and Hess grades 1–3 just prior to aneurysm repair and 53% had their ruptured aneurysm in the frontal circulation (anterior cerebral arteries and pericallosal arteries).
Good versus poor global cognitive function
Based on their GCII score, 29 patients (57%) were classified as having poor cognitive function, whereas 22 patients (43%) had a good cognitive function. The mean GCII for the poor function patients was 0.93 (SD 0.09) and 0.43 (SD 0.25), respectively, for the good function patients. Results for the good and poor function patients are displayed in Table 2. There were no significant differences between the two groups in terms of gender, age, education, Hunt and Hess score, Glasgow Coma Scale, or aneurysm location (see Table 3). In the poor cognitive function group, there were 11 males and 18 females with an average of 49 years (range 25–76 years) and 13 years of education (range 8–20 years). In the good cognitive function group, there were 10 males and 12 females with an average age of 51 years (range 26–64 years) and 13 years of education (range 9–20 years). In the poor cognitive function group, most neuropsychological tests fell in the severe cognitive deficit range, with the exception of an attention/working memory test (digit span) which fell in the moderate deficit range.
In the good cognitive function group, the neuropsychological test scores fell in the normal to moderate deficit range. Tests of attention/working (digit span) and inhibition (color-word interference test condition 3) were not affected while long-term memory (BVMT delayed recall) and some tests of fine- and psychomotor speed (grooved pegboard and color-word interference test condition 1) were moderately affected in the good cognitive function group (see Table 2).
Modified Rankin scale (mRS) versus cognitive functioning
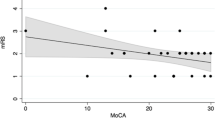
Based on the mRS on the date of cognitive testing, 19 out of 39 patients that were scored as mRS 1 or 2 (i.e., good functional outcome) had GCII scores in the poor range (at or above 0.75), i.e., mRS failed to detect a significant deficit in almost half of the patients that appeared to be in a good condition. The 12 patients that were scored with a mRS of 3 or 4 (i.e., poor functional outcome) had a GCII score at 0.75 or above in 10 of the instances. There was hence a significant mismatch between mRS and GCII apprehended function (Fig. 1).
Good and poor functional outcome on mRS versus GCII. Figure text: Dark symbol visualizes mismatch between functional outcome in terms of mRS (good function 0–2) versus global cognitive function (good < 0.75). One symbol may represent multiple data points, so that there was a mismatch in 19 out of 39 patients with mRS 0–2
Medical predictors for global cognitive function
Medical predictors for cognitive good and poor function groups, as well as the relationship to the GCII, are shown in Table 3. The most powerful predictors of poor cognitive function were linked to acute hydrocephalus, in terms of need for larger volumes CSF drained and larger ventricle size (large Evans index). The amount of intraventricular blood (LeRoux), draining over 2000 ml of CSF, having acquired a new cerebral infarction and a large Evans index at any point of time was related to poor cognitive functioning. Further, the results from a multivariate regression analysis (see Table 4) with these four variables as independent variables showed that having acquired a new cerebral infarction and needing CSF drainage in excess of 2000 ml were the strongest predictors for poor cognitive function. The latter increased the risk of poor cognitive function almost 6-fold, whereas acquiring a new cerebral infarction 11-folded the risk of poor cognitive function in the acute phase of aSAH. Although not included in the multiple regression analysis, our results also indicate that the cognitively poor outcome patients spent more time in the ICU and in general needed neurosurgical care for a longer time period than the cognitively good functioning patients (see Table 3). Complications during securing of the aneurysm were as follows: Thirty-six (70%) of the patients had no complications, 5 (10%) had thromboembolic events during coiling, another 3 (6%) had coil protrusion, in 3 (6%) the aneurysm ruptured during clipsing, and the remaining four patients had either aneurysm perforation during coiling or blood vessel occlusion during clipsing or during coiling. Most of these complications were not related to neurological sequels.
Discussion
The majority of patients (57%) had a severe general cognitive deficit in the acute phase after aSAH that was not reflected in their mRS in about half of the cases. Correspondingly, the remaining aSAH patients had a good cognitive function with only mild to moderate cognitive deficits in memory and psychomotor speed. Acute hydrocephalus and aSAH-acquired cerebral infarction were the strongest predictors of poor cognitive function.
Cognitive function in the acute phase after aSAH
Aneurysmal subarachnoid hemorrhage can have a devastating effect on a person’s life and many struggle in the months and years after an aSAH. Research on outcome in the chronic phases after aSAH has shown that cognitive deficits are a major contributor to reduced quality of life [19]. Increased knowledge about the nature of these cognitive deficits and early detection of the cognitive problems are therefore of vital importance in order to plan further rehabilitation of aSAH patients.
Patients with a good cognitive function in the acute phase after aSAH in our study showed for the most part normal to mild deficits on the neuropsychological tests, and only delayed memory and some measures of fine- and psychomotor functions were moderately reduced. On the other hand, the patients with poor cognitive function had a severe global cognitive deficit with scores mostly falling well below two standard deviations from the expected mean. Delayed memory was affected in both groups. This is in line with previous research on aSAH in the chronic phase, which has shown that memory is one of the most frequently occurring and most debilitating cognitive problems [19, 24]. Further, the problems with fine- and psychomotor speed is also in accordance with previous research on aSAH patients [13] and has shown to improve over time [9]. Overall, the levels of cognitive deficits seen in the good outcome group is therefore consistent with research on aSAH patients in the chronic phase with mild to moderate cognitive deficits especially in memory and psychomotor speed [28]. mRS failed to identify the poor cognitive function in about half of the patients with a GCII at or above 0.75 and hence does not seem to be adequate in evaluating cognitive deficits in the acute phase after aSAH. Neuropsychological screening should therefore be implemented as a complement to the mRS in the acute phase after aSAH.
The impact of acute hydrocephalus on cognitive function in the acute phase
The present study shows that there is a large group of patients with severe global deficits in the acute phase, which seems to be related to acute hydrocephalus and acquisition of a new cerebral infarction. Similar findings were also reported in the study by Hütter [14]. They found acute hydrocephalus to be a significant predictor for poor cognitive function and stated that “even a slight and temporary ventricular dilation had significant neuropsychological effects.” This aspect is why we considered the largest Evans index at any point of time during the primary stay in our study. Any stretch of structures adjacent to the frontal parts of the ventricular system may cause damage leading to cognitive deficits. Animal studies [37] have suggested that ventriculomegaly leads to impairments in widespread regions of the CNS, especially the corpus callosum, periventricular white matter, fornix, internal and external capsules, and other white matter regions. Research on rats has shown that the animals are more susceptible to negative behavioral effects during the early stages of ventricular enlargement [35]. The reason for the more pervasive cognitive impairment at earlier time points may be the result of rate of change over time. It has been shown that serial lesions may cause less cognitive impairment than single-stage lesions and that slow expanding lesions produce less functional loss than rapid developing ones [7, 16], similar to the situation in acute hydrocephalus in aSAH. The swift intracranial volume increase caused by acute ventriculomegaly should not be equated with the finding of increased CSF spaces in the chronic phases after aSAH, where large CSF spaces can be a sign of diffuse brain atrophy. In a study by Bendel [2], they found that enlarged CSF spaces correlate with cognitive deficits after aSAH. In their study, the group of patients with at least one neuropsychological deficit had increased modified cella media index (mCMI) and the cerebral spinal fluid (CSF)/total intracranial volume (ICV) ratios were higher compared to those patients without any neuropsychological deficits. In line with the higher CSF/ICV ratios, the aSAH patients with neuropsychological deficits showed lower gray matter/ICV ratios and in a separate analysis of each cognitive domain in the Bendel study [2], they found that higher mCMIs and CSF/ICV ratios were detected in patients with a cognitive deficit in the chronic phase. Ventriculomegaly after aSAH have been reported in the literature [5, 17, 32], and ventricular dilation after aSAH is usually related to hydrocephalus; however, ventricular dilation is also often combined with sulcal dilation, causing findings similar to the diffuse brain atrophy detected in patients with severe TBI [1]. Ventricular and sulcal enlargement, together with reduced gray matter volumes, after aSAH may therefore indicate general atrophy rather than hydrocephalus. Therefore, the data of Bendel et al. [2] cannot determine if the cognitive deficits were the result of an atrophic process or if these deficits already were present in the acute state—prior to any atrophy taking place. Our data support that this may be the case, and, if so, in order to prevent poor cognitive functioning, it would be of outmost importance to aggressively treat acute hydrocephalus by immediate CSF diversion not allowing vast expansion of the ventricular system at any point of time.
The impact of cerebral infarction on cognitive function in the acute phase
Acute hydrocephalus and new ischemic lesions causing poor cognitive function in the acute phase are in line with previous research that has concluded that the perhaps strongest association between cognitive functioning and medical variables has been found in relation to cerebral edema [19]. This association is explained through suggesting that global edema may be a manifestation of transient global ischemia related to elevated intracranial pressure at the time of the bleeding, resulting in microvascular injury, impaired autoregulation, and rebound hyperemia. This may even apply to patients in good clinical grade (Hunt and Hess grades 1, 2, and 3) like most of our patients. A few patients acquired lesions mainly in the frontal lobes during aneurysm repair, mostly during endovascular aneurysm repair. The additional trauma of surgical aneurysm repair had no detectable impact on cognitive functioning, as there were more individuals with surgical aneurysm repair in the cognitive good functioning group.
Cerebral vasospasm is one of the most feared complications to aSAH, potentially causing lethal multiple delayed ischemic events. Some studies have looked at the effect of vasospasms on cognitive functioning and although early studies found an association [20, 29, 34], the improved treatment of vasospasms using nimodipine and hemodilution hypertensive therapy have led to studies not finding any associations [4, 11]. Unfortunately, it is therefore not yet clear what effect vasospasm has on cognitive functions, because vasospasms can occur at any arterial position in the brain and hence affect any number of brain functions. Furthermore, the definition of vasospasm is not straightforward as vasospasm can be expressed as a radiological finding with or without clinical correlate or as delayed cerebral ischemia, further obscuring its relevance to functional deficits. Severe cerebral vasospasm was rare in the patients we presently investigated, but still, there was a trend to more vasospasm in the cognitive poor functioning group (p = 0.07). The prevalence of vasospasm is expected to be higher in high-grade aSAH (Hunt and Hess grades 4 and 5) and needless to say, a strong focus on preventing, monitoring, and treating cerebral vasospasm would be mandatory in order to provide a best possible cognitive outcome in this especially vulnerable sub-group of aSAH.
Strengths and limitations
Among the cohort of aSAH patients, there was a large fraction of patients that were unable to complete neuropsychological testing in the acute phase. This is mostly due to a large percentage of poor-grade aSAH patients treated. Our material is hence biased towards good-grade aSAH patients. However, our results are probably applicable to the entire specter of aSAH severity. The time from ictus to cognitive assessment varied due to patients in a poorer clinical condition being hospitalized at the neurosurgical ward for a longer time. A strength is that our neurosurgical department handles relatively large aSAH volumes (120–150 aSAH/year) and has very standardized treatment guidelines. Surgical and endovascular aneurysm repair is available 24 h every day and is performed by dedicated vascular teams. Furthermore, our patients are treated at a neurointermediate ward with a nursing staff that is especially trained and highly dedicated to the aSAH group. This contributed to actually being able to recruit all aSAH patients capable of undergoing neuropsychological testing for the study.
Presently, the levels of cognitive deficits are consistent with research on aSAH patients in the chronic phase with mild to moderate cognitive deficits especially in memory and psychomotor speed. However, the predictive value of cognitive function in the acute phase for cognitive performance in the chronic phase remains unknown and needs to be explored in future research.
Conclusion
More than half of patients (57%) have severe cognitive deficits in the acute phase after aSAH. mRS should be combined with neuropsychological screening in the acute phase after aSAH to get a more accurate description of the patients’ disabilities. Acute hydrocephalus and aSAH-acquired cerebral infarction are the strongest predictors of poor cognitive function in the acute phase.
References
Bendel P, Koivisto T, Niskanen E, Kononen M, Aikia M, Hanninen T, Koskenkorva P, Vanninen R (2009) Brain atrophy and neuropsychological outcome after treatment of ruptured anterior cerebral artery aneurysms: a voxel-based morphometric study. Neuroradiology 51:711–722. https://doi.org/10.1007/s00234-009-0552-5
Bendel P, Koivisto T, Aikia M, Niskanen E, Kononen M, Hanninen T, Vanninen R (2010) Atrophic enlargement of CSF volume after subarachnoid hemorrhage: correlation with neuropsychological outcome. AJNR Am J Neuroradiol 31:370–376. https://doi.org/10.3174/ajnr.A1804
Benedict R (1997) Brief visuospatial memory test-revised: professional manual. Psychological Assessment Resources, Inc, Lutz
Berry E, Jones RA, West CG, Brown JD (1997) Outcome of subarachnoid haemorrhage. An analysis of surgical variables, cognitive and emotional sequelae related to SPECT scanning. Br J Neurosurg 11:378–387
Dehdashti AR, Rilliet B, Rufenacht DA, de Tribolet N (2004) Shunt-dependent hydrocephalus after rupture of intracranial aneurysms: a prospective study of the influence of treatment modality. J Neurosurg 101:402–407. https://doi.org/10.3171/jns.2004.101.3.0402
Delis D, Kaplan K, Kramer J (2001) Delis and Kaplan executive function system. Harcourt Brace & Co, San Antonio
Finger S, Walbran B, Stein DG (1973) Brain damage and behavioral recovery: serial lesion phenomena. Brain Res 63:1–18
Fisher CM, Roberson GH, Ojemann RG (1977) Cerebral vasospasm with ruptured saccular aneurysm--the clinical manifestations. Neurosurgery 1:245–248
Haug T, Sorteberg A, Sorteberg W, Lindegaard KF, Lundar T, Finset A (2007) Cognitive outcome after aneurysmal subarachnoid hemorrhage: time course of recovery and relationship to clinical, radiological, and management parameters. Neurosurgery 60:649–656; discussion 656-647. https://doi.org/10.1227/01.neu.0000255414.70807.a0
Heaton RK, Grant I, Matthews C (1991) Comprehensive norms for an expanded halstead-reitan neuropsychological battery: demographic corrections, research findings, and clinical applications. Psychological Assessment Resources, Odessa
Hillis AE, Anderson N, Sampath P, Rigamonti D (2000) Cognitive impairments after surgical repair of ruptured and unruptured aneurysms. J Neurol Neurosurg Psychiatry 69:608–615
Hunt WE, Hess RM (1968) Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 28:14–20. https://doi.org/10.3171/jns.1968.28.1.0014
Hütter B (2000) Neuropsychological Sequelae of subarachnoid hemorrhage and its treatment. Springer Verlag, Wien. https://doi.org/10.1007/978-3-7091-6327-6
Hutter BO, Kreitschmann-Andermahr I, Gilsbach JM (1998) Cognitive deficits in the acute stage after subarachnoid hemorrhage. Neurosurgery 43:1054–1065
Investigators ISUIA (1998) Unruptured intracranial aneurysms--risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med 339:1725–1733. https://doi.org/10.1056/nejm199812103392401
Isseroff A, Leveton L, Freeman G, Lewis ME, Stein DG (1976) Differences in the behavioral effects of single-stage and serial lesions of the hippocampus. Exp Neurol 53:339–354
Jartti P, Karttunen A, Isokangas JM, Jartti A, Koskelainen T, Tervonen O (2008) Chronic hydrocephalus after neurosurgical and endovascular treatment of ruptured intracranial aneurysms. Acta Radiol 49:680–686. https://doi.org/10.1080/02841850802050754
Jennett B, Bond M (1975) Assessment of outcome after severe brain damage. Lancet 1:480–484
Kreiter KT, Copeland D, Bernardini GL, Bates JE, Peery S, Claassen J, Du YE, Stern Y, Connolly ES, Mayer SA (2002) Predictors of cognitive dysfunction after subarachnoid hemorrhage. Stroke 33:200–208
Larsson C, Ronnberg J, Forssell A, Nilsson LG, Lindberg M, Angquist KA (1989) Verbal memory function after subarachnoid haemorrhage determined by the localisation of the ruptured aneurysm. Br J Neurosurg 3:549–560
Le Roux PD, Elliott JP, Newell DW, Grady MS, Winn HR (1996) Predicting outcome in poor-grade patients with subarachnoid hemorrhage: a retrospective review of 159 aggressively managed cases. J Neurosurg 85:39–49. https://doi.org/10.3171/jns.1996.85.1.0039
Levin HS, O'Donnell VM, Grossman RG (1979) The Galveston orientation and amnesia test. A practical scale to assess cognition after head injury. J Nerv Ment Dis 167:675–684
Lindekleiv HM, Njolstad I, Ingebrigtsen T, Mathiesen EB (2011) Incidence of aneurysmal subarachnoid hemorrhage in Norway, 1999-2007. Acta Neurol Scand 123:34–40. https://doi.org/10.1111/j.1600-0404.2010.01336.x
Ogden JA, Mee EW, Henning M (1993) A prospective study of impairment of cognition and memory and recovery after subarachnoid hemorrhage. Neurosurgery 33:572–586 discussion 586-577
Orbo M, Waterloo K, Egge A, Isaksen J, Ingebrigtsen T, Romner B (2008) Predictors for cognitive impairment one year after surgery for aneurysmal subarachnoid hemorrhage. J Neurol 255:1770–1776. https://doi.org/10.1007/s00415-008-0047-z
Passier PE, Visser-Meily JM, van Zandvoort MJ, Post MW, Rinkel GJ, van Heugten C (2010) Prevalence and determinants of cognitive complaints after aneurysmal subarachnoid hemorrhage. Cerebrovasc Dis 29:557–563. https://doi.org/10.1159/000306642
Rankin J (1957) Cerebral vascular accidents in patients over the age of 60. III. Diagnosis and treatment. Scott Med J 2:254–268
Ravnik J, Starovasnik B, Sesok S, Pirtosek Z, Svigelj V, Bunc G, Bosnjak R (2006) Long-term cognitive deficits in patients with good outcomes after aneurysmal subarachnoid hemorrhage from anterior communicating artery. Croat Med J 47:253–263
Richardson JT (1989) Performance in free recall following rupture and repair of intracranial aneurysm. Brain Cogn 9:210–226
Samra SK, Giordani B, Caveney AF, Clarke WR, Scott PA, Anderson S, Thompson BG, Todd MM (2007) Recovery of cognitive function after surgery for aneurysmal subarachnoid hemorrhage. Stroke 38:1864–1872. https://doi.org/10.1161/strokeaha.106.477448
Santiago-Ramajo S, Katati MJ, Perez-Garcia M, Coin-Mejias MA, Vilar-Lopez R, Caracuel-Romero A, Arjona-Moron V (2007) Neuropsychological evaluation of the treatments applied to intracranial aneurysms in a Spanish sample. J Clin Exp Neuropsychol 29:634–641. https://doi.org/10.1080/13803390600879024
Sethi H, Moore A, Dervin J, Clifton A, MacSweeney JE (2000) Hydrocephalus: comparison of clipping and embolization in aneurysm treatment. J Neurosurg 92:991–994. https://doi.org/10.3171/jns.2000.92.6.0991
Sorteberg W, Slettebo H, Eide PK, Stubhaug A, Sorteberg A (2008) Surgical treatment of aneurysmal subarachnoid haemorrhage in the presence of 24-h endovascular availability: management and results. Br J Neurosurg 22:53–62. https://doi.org/10.1080/02688690701593553
Stenhouse LM, Knight RG, Longmore BE, Bishara SN (1991) Long-term cognitive deficits in patients after surgery on aneurysms of the anterior communicating artery. J Neurol Neurosurg Psychiatry 54:909–914
Toma AK, Holl E, Kitchen ND, Watkins LD (2011) Evans’ index revisited: the need for an alternative in normal pressure hydrocephalus. Neurosurgery 68:939–944. https://doi.org/10.1227/NEU.0b013e318208f5e0
Wechsler D (2008) Wechsler adult intelligence scale, fourth edn. Pearson, San Antonio
Williams MT, Braun AA, Amos-Kroohs RM, McAllister JP 2nd, Lindquist DM, Mangano FT, Vorhees CV, Yuan W (2014) Kaolin-induced ventriculomegaly at weaning produces long-term learning, memory, and motor deficits in rats. Int J Dev Neurosci 35:7–15. https://doi.org/10.1016/j.ijdevneu.2014.02.002
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Comments
Neuropsychological sequelae of cerebral injuries are not always adequately identified by the treating personnel. This may be even more true if the underlying cause is an acute and potentially life-threatening situation, such as aneurysmal subarachnoid hemorrhage. Typical instruments used to rate neurological disability in these situations, such as modified Rankin scale or extended Glasgow outcome scale, are also not very sensitive to cognitive symptoms, if the gross neurological functioning of the patient is largely intact—especially when assessed at the time of discharge. This study by Haug Nordenmark et al. raises awareness of this issue. Importantly, they demonstrate that significant proportion of aSAH survivors with “good” outcome based on mRS score has considerable neurocognitive problems at the time of discharge. It is our responsibility as treating physicians of these patients to try and identify those patients most in need of neuropsychological rehabilitation and not let them fall through the safety net without adequate support.
Aki Laakso
Helsinki, Finland
This article is part of the Topical Collection on Vascular Neurosurgery - Aneurysm
Rights and permissions
About this article
Cite this article
Haug Nordenmark, T., Karic, T., Sorteberg, W. et al. Predictors of cognitive function in the acute phase after aneurysmal subarachnoid hemorrhage. Acta Neurochir 161, 177–184 (2019). https://doi.org/10.1007/s00701-018-3760-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-018-3760-0