Abstract
Gleichenia boryi is a poorly known species of Gleicheniaceae endemic to Madagascar and La Réunion Island. Although Kunze pointed out in his description that this fern was distinct from other Gleicheniaceae in its leaf morphology, the generic relationships of this fern have not been investigated until now. Taking advantage of DNA sequences obtained from a sample of this species obtained in La Réunion Island and a sample of the type species Gl. polypodioides, we tested the hypothesis that the morphological distinction reflects the phylogenetic isolation of Gl. boryi. By reconstructing the phylogenetic relationships and divergence time estimates, we were able to show that this fern is not closely related to Gleichenia s.s. and represents a distinct lineage segregated from other Gleicheniaceae since the Cretaceous. Thus, a new generic name Rouxopteris (gen. nov.) and a new combination R. boryi (comb. nov.) are introduced to improve the generic classification of this family. We also discussed the evolutionary history of the Gleicheniaceae in the context of the Cretaceous terrestrial revolution.
Similar content being viewed by others
Introduction
Our understanding of the phylogeny and classification of Gleicheniaceae C.Presl (Presl 1825) has considerably improved in recent years, mainly as a result of DNA sequence-based phylogenetic analyses (e.g., Schuettpelz and Pryer 2007; Perrie et al. 2007, 2012; Li et al. 2010; Shu et al. 2017). Older classifications often recognized only two genera, Dicranopteris Bernh. and Gleichenia Sm. (Smith 1793; Bernhardi 1805; Holttum 1957a, b, 1959; Tryon and Tryon 1982), whereas later published classifications recognized six genera Dicranopteris, Diplopterygium (Diels) Nakai, Gleichenia, Gleichenella Ching, Sticherus C.Presl, and Stromatopteris Mett. (Presl 1836; Mettenius 1861; Ching 1940; Nakai 1950; Smith et al. 2006, 2008; Christenhusz et al. 2011; PPG1 2016). The increase in genera was the result of inclusion of Stromatopteris previously considered to represent a separate family (Bierhorst 1968), and also by segregation of Diplopterygium and Sticherus from Gleichenia and Gleichenella from Dicranopteris (Ching et al. 1959; Kramer 1990). Some of these segregates were recognized by Holttum (Holttum 1957a, b, 1959) as subgenera of Dicranopteris and Gleichenia, respectively. They were subsequently accepted as separate genera in the latest pre-phylogenetic analyses (Ching 1978; Kramer 1990). Thus, all currently accepted genera were all proposed based on morphological diagnostic features and found to be consistent with the phylogeny inferred using DNA sequence data (Schuettpelz and Pryer 2007).
The changes in the classification reduced the genus Gleichenia to a small genus of about 10 species sharing highly similar leaf morphology (Holttum 1957a; Kramer 1990; Shaw and Ranker 2011). The majority of species belonging to this genus have pseudo-dichotomously branched leaves with 2 to 4 tiers of opposite pinnae and a dormant vegetative bud between the terminal pair of pinnae. The ultimate segments contain a single sorus (Kramer 1990). However, this generic description does not encompass some poorly known species occurring in Madagascar and the Mascarene Islands. Kunze (1844) pointed out in his description of Gleichenia boryi Kunze that it is distinct from other Gleicheniaceae in its leaf morphology (Fig. 1). Instead of the pseudo-dichotomous branching with terminal dormant buds, this fern possesses bi-pinnatifid leaves without dormant buds but a well-defined rachis. Dormant buds are also absent in other species of Gleichenia such as Gl. abscida Rodway (Rodway 1903) from Tasmania and Gl. elongata Baker (Baker 1901) from East Africa (Chinnock and Bell 1998; Perrie et al. 2012). However, some of these species still show pseudo-dichotomous arrangements of the pinnae, whereas the pinnae of Gl. boryi are not placed opposite to each other and thus they do not form tiers of pinnae. In turn, this species shares other features either with Gleichenia or other Gleicheniaceae such as the gleichenioid sporangium and long-creeping rhizomes. The shape of the ultimate segments resembles species belonging to Gleichenia. Other studies pointed out that the vascular tissue organization in the petiole is less similar to other species of Gleichenia than to those found in Sticherus (Chrysler 1944). However, close relationships to Sticherus are not supported by the occurrence of trilete spores in Gl. boryi because all species of Sticherus as far as known possess monolete spores (Kramer 1990; Tryon and Lugardon 1991). Thus, the information provided by the leaf morphology requires further investigation and the unusual leaf shape may be explained as a transition from the leaf shapes shown by species such as Afromadagascan Gl. polypodioides (L.) Sm. (Smith 1793), which is the type of the genus (Mildbread 1934). In recent treatments of Gleichenia, the unusual leaf morphology of Gl. boryi has received little attention, which may be mainly caused by its restricted occurrence in the Mascarene Islands and Madagascar (Christensen 1932a, b; Tardieu-Blot 1982; Badré 2008; Roux 2009; Grangaud 2010). The putative segregate Gl. madagascariensis C.Chr. (Christensen 1932b, b) has been accepted as a variety of Gl. boryi in recent taxonomic treatments (Badré 2008; Roux 2009).
In this study, we address the phylogenetic relationships of Gleichenia boryi to other gleichenioid ferns especially Gl. polypodioides. This Afromadagascan species is distinct in its typical pseudo-dichotomous gleichenioid leaf morphology from Gl. boryi and it is in turn the type species of Gleichenia. Using sequences of the chloroplast rbcL gene, we reconstructed a global phylogenetic framework that includes representatives of all accepted genera and is rooted with the putative closest relatives Dipteridaceae and Matoniaceae (Schuettpelz and Pryer 2007). Given the unusual leaf morphology, the relationships of Gl. boryi may be of crucial importance for the interpretation of the phylogenetic relationships of Cretaceous Gleicheniaceae fossils such as Boodlepteris turoniana Gandolfo (Gandolfo et al. 1997) and Gl. chaloneri Herendeen & Skog (Herendeen and Skog 1998). We aim to create a scenario of the divergence of extant gleichenioid ferns with focus on the origin of Gl. boryi. By doing so, we aim to establish the first comprehensive phylogeny to reconstruct the generic relationships and early divergence of the Gleicheniaceae crown lineage, with a focus on the origin of Gl. boryi.
Materials and methods
Leave material dried in silica of all Gleicheniaceae species—Dicranopteris cadetii Tardieu (Tardieu-Blot 1982), Dic. linearis (Burm.f.) Underw. (Underwood 1907), Gleichenia boryi, Gl. polypodioides, Sticherus flagellaris (Bory ex Willd) Ching (Ching 1940)—occurring in the Mascarene Islands were obtained during fieldwork. Genomic DNA was extracted from these specimens using CTAB (Doyle and Doyle 1987) and used to amplify rbcL sequences with PCR protocols and primers as described in Hennequin et al. (2014). The PCR products were cleaned and used to generate DNA sequences via an ABI 3730xl DNA analyses and the BigDye chemistry (Applied Biosystems, Carlsbad, CA, USA). The newly generated sequences were assembled and edited using Sequencher 5.2. (Gene Codes Corporation, Ann Arbor, MI, USA). All sequences were used in a previous study (Hennequin et al. 2014) and deposited at GenBank (see Fig. 2 for GenBank accession numbers). These sequences were combined with rbcL sequences available at GenBank (ncbi.nlm.nih.gov). We downloaded all rbcL sequences available for Gleicheniaceae and the two most closely related families Dipteridaceae and Matoniaceae. The original dataset was reduced to a single representative per species if sequences with more than one specimen were available. The final dataset included 37 species and is available as a Supplementary File at the website of Plant Systematics and Evolution. Four species of Matoniaceae—two species of Matonia R.Br. ex Wall. (Wallich 1829), two species of Phanerosorus Copel. (Copeland 1909)—and five species of Dipteridaceae—three species of Cheiropleuria C.Presl (Presl 1851), two species of Dipteris Reinw. (1825)—were assigned as outgroup taxa. The ingroup comprised all six genera of Gleicheniaceae: five species of Dicranopteris, eight species of Diplopterygium, five species of Gleichenia, one species of Gleichenella, seven species of Sticherus and one species of Stromatopteris. The dataset included the type species of all genera proposed except the type species of Hicriopteris C.Presl (Presl 1851), which is currently accepted as Dic. speciosa (C.Presl) Holttum (Holttum 1957b) (see Table 1). The alignment was assembled and adjusted using Mesquite 3.50 (Maddison and Maddison 2018). The incomplete ends of the sequences were trimmed reducing the final dataset from 1209 characters to 858 characters. Taxonomic treatments of the Afromadagascan species followed Roux (2009), whereas the treatment of Chinese species follows Jin et al. (2013). Thus, Sti. laevigatus (Willd.) C.Presl (Presl 1836) was treated as a synonym of Sti. truncatus (Willd.) Nakai (Nakai 1950) and Dic. pedata (Houtt.) Nakaike (Nakaike 1975) was treated as distinct from Dic. linearis (Burm.f.) Underw. (Underwood 1907).
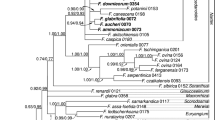
Consensus phylogram obtained by Bayesian inference of phylogeny using rbcL sequences. Values above branches correspond to posterior values, whereas values below branches correspond to bootstrap values obtained in ML analyses. The topology of the consensus phylogram is identical with the topology found for the most likely tree in ML analyses. Values given as: ** correspond to p = 1.00 and BS = 100%, respectively; * correspond to p ≥ 0.95 and BS ≥ 75%, respectively. No lower values are shown except for the node linking Gleichenia (Rouxopteris) boryi to the Dicranopteris-Gleichenella-Diplopterygium clade (arrow p value/bootstrap value)
Phylogenetic analyses were carried out using maximum parsimony (MP) as implemented in PAUP (Swofford 2003), maximum likelihood (ML) as implemented in RaxML (Stamatakis 2006) and Bayesian inference of phylogeny (BI) using MrBayes 3.2 (Ronquist et al. 2012). JModelTest 2 (Darriba et al. 2012) was used to identify the model of sequence evolution to be implemented in the ML and BI analyses. All analyses were carried out using standard default options. Additionally, Neighbor-Net analyses (Bryant and Moultan 2004) were carried out using SplitsTree 4 (Huson and Bryant 2006). These analyses were carried out using LogDet distances (Lockhart et al. 1994), equal angle projection and filtering of splits using least square and four dimension options. Divergence time estimates were obtained using BEAST 1.75 (Drummond et al. 2012). Analyses were carried out under the assumption of a constant clock and relaxed lognormal clock but final analyses were based on a relaxed clock model calibrated with the estimate for the crown node age of Gleicheniales (split of Gleicheniaceae and sister lineages) as estimated by Schuettpelz and Pryer (2009). The node age of 262.2 Ma was used as a lognormal constraint because Schuettpelz and Pryer (2009) did not provide confidence intervals. Final estimates were carried out using a Birth–Death tree parameter. We also considered several Cretaceous fossils (Gandolfo et al. 1997; Herendeen and Skog 1998), but their assignment requires careful reinvestigation of character state changes as indicated by our findings concerning the relationship of Gleichenia boryi. Thus, we decided to use the fossil evidence and divergence time estimates as independent evidence to explore the reliability of the estimated diversification times. Gamma value (Pybus and Harvey 2000) and related statistics were estimated using TreeStat 1.7.5 of the BEAST package.
Results
Consistent with the unique leaf morphology, Gleichenia boryi was found to form an isolated lineage and was not nested within the Gleichenia clade (Fig. 2). The family Gleicheniaceae were divided into two main clades. One clade comprised Gleichenia, Sticherus and Stromatopteris, whereas the other clade consisted of Dicranopteris, Diplopterygium and Gleichenella. Rouxopteris (Gleichenia) boryi was found to be the sister to the later clade. However, several of the branches connecting the main clades of Gleicheniaceae showed low bootstrap values (< 75%) and posterior values (< 0.95). Neighbor-Net analyses recovered Gl. boryi as a separate lineage with splits connecting it to members of both main clades recovered in phylogenetic analyses (Fig. 3). Divergence time estimates suggested an initial radiation of the Gleicheniaceae crown group (128.7 to 93.1 Ma) coinciding with the Cretaceous Terrestrial Revolution (KTR), whereas the majority of modern genera showed a divergence dating back only to the Miocene, e.g., Dicranopteris 16.7 to 4.4 Ma; Diplopterygium without Dip. bancroftii 13.6 to 5.1 Ma, Gleichenia 18.9 to 5.9 Ma and Sticherus without Sti. truncatus (Sti. laevigatus) 18.0 to 7.8 Ma (Fig. 4). The segregation of Rouxopteris (Gleichenia) boryi dated back to about 118.8 to 81.3 Ma and thus it was somewhat older than the split of Stromatopteris and Gleichenia (93.6 to 58.7 Ma), the split of Diplopterygium and the Dicranopteris-Gleichenella clade (90.5 to 57.0 Ma) and the onset of the diversification of Sticherus (97.2 to 63.3 Ma).
Discussion
This study provides one of the most comprehensive phylogenetic analyses of the Gleicheniaceae based on chloroplast DNA sequences. The reconstructed phylogenetic relationships are consistent with the generic classification (Smith et al. 2006; PPG 1 2016) with the exception of the position of Rouxopteris (Gleichenia) boryi which forms a separate clade not close to any other gleichenioid ferns. Besides this obvious difference, the generic classification of the genus Sticherus requires further attention because of the deep divergence between Sti. truncatus and the remaining sampled species of this genus. The later clade showed rather low divergence despite the sampling including South American species [Sti. bifidus (Willd.) Ching (Ching 1940), Sti. cryptocarpus (Hook.) Ching (Ching 1940), and Sti. palmatus (W.Schaffn. ex E.Fourn.) Copel (Copeland 1947)], Afromadagascan [Sti. flagellaris] and Australasian species [Sti. cunninghamii (Heward ex Hook.) Ching (Ching 1940) and Sti. flabellatus (R.Br.) H.St.John (John 1942)].
Early diversification of Gleicheniaceae
The discovery of the separation of Rouxopteris (Gleichenia) boryi sheds new light on the early diversification of Gleicheniaceae, an old lineage of leptosporangiate ferns. The results are consistent with previous reports indicating an establishment of the stem of Gleicheniaceae in the early Mesozoic—including the sister relationship of the stem group of Gleicheniaceae to the clade comprising Dipteridaceae and Matoniaceae. The results also show a likely rapid radiation of the crown group Gleicheniaceae during the Cretaceous Terrestrial Revolution (KTR), which leads to the establishment of the genera recognized in current classifications with the exception of the segregation of two more recent genera Dicranopteris and Gleichenella. The obtained age estimates are highly consistent with estimates published in previous studies (Pryer et al. 2004; Schuettpelz and Pryer 2009; Testo and Sundue 2014). Thus, Gleicheniaceae joins the group of fern lineages that diversified in the shadow of angiosperms (Schneider et al. 2004). The hypothesis of a rapid diversification during the Cretaceous is consistent with the fossil record because fossils assignable to the crown group date back only to the Cretaceous (Gandolfo et al. 1997; Herendeen and Skog 1998; Skog 2001). In this scenario, Triassic and Jurassic putative Gleicheniaceae are interpreted as stem group representatives.
In turn, these results have some interesting implications. Firstly, the results suggest a previously unrecognized pattern during the Cretaceous Terrestrial Revolution. Lineage replacement and/or increased speciation rate were not restricted to plant lineages growing inside of forests but also affected lineages that dominated open, disturbed habitats such as Gleicheniaceae (Kramer 1990). Secondly, the recovered phylogenetic relationships suggest that rather recent diversification (Miocene) events lead to the extant diversity of the genera Dicranopteris, Diplopterygium, Gleichenia and probably Sticherus (Fig. 4).
The interpretation of these results requires taking into account the taxonomic coverage. Current estimates of the extant species diversity of Gleicheniaceae recognize 157 species (PPG1 2016) of which 27 species were represented in our study (17%). Besides the two monotypic genera Gleichenella and Stromatopteris, the remaining genera were sampled with uneven density, namely Dicranopteris (5 out of ca. 20 spp.; 25%), Diplopterygium (8 out of 25 spp.; 32%), Gleichenia (5 out of 15 spp.; 33%), and Sticherus (7 out of 95 spp.; 7%). The lack of sampling density is arguably of special concern for Sticherus because this is not only the most species-rich genus of Gleicheniaceae comprising about 61% of the family diversity but also our results show evidence for an deep divergence between the only Sticherus species occurring in China, Sti. truncatus, and the remaining sampled species diversity of the genus. This is in contrast to the low divergence among the remaining six species despite them representing a wide area including South America, Afromadagascar and Australasia. To evaluate this pattern, a denser sampling of the genus Sticherus is required, arguably with focus on species occurring in Malaysia such as Sti. hirtus (Blume) Ching (Ching 1940; Holttum 1959). In conclusion, the recovered patterns indicate a rather dynamic diversification of Gleicheniaceae in the late Mesozoic and Cenozoic comparable in its timing to derived ferns despite the lower species diversity (Schneider et al. 2004; Schuettpelz and Pryer 2009).
The phylogenetic position of Rouxopteris (Gleichenia) boryi also provides new insights into the evolution of leaf morphology of gleichenioid ferns. The absence of pseudo-dichotomous branching in this species supports a scenario in which the putative apomorphy of the family, pseudo-dichotomous leaf morphology, has been lost independently more than once. Thus, this feature is not unique to Stromatopteris. Instead, Rouxopteris (Gleichenia) boryi is a further example that this character is less conserved in Gleicheniaceae than assumed in taxonomic descriptions (Kramer 1990). Another species without pseudo-dichotomous branching, Sticherus simplex (Desv.) Ching (Ching 1940), is also widely ignored in the taxonomic literature although this species is not rare in Andean paramos (Gonzales and Kessler 2011). Other examples such as Gl. abscida and Gl. elongata (Perrie et al. 2012) require further investigation because their relationships need to be considered as uncertain. They may be either part of Gleichenia s.s. or related to Gl. boryi. Most interestingly, this discovery suggests the need to restudy the phylogenetic position of Cretaceous gleichenioid fossils. Previous studies (Gandolfo et al. 1997; Herendeen and Skog 1998) did not consider all the lineages that we recovered in the phylogenetic analyses of Gleicheniaceae. Thus, their inferences need to be re-evaluated carefully. For example, new insights provided no support for a division of the family into taxa with and without scales. Instead, scales appeared to be the ancestral feature and the reduction to hairs is the derived state restricted to the clade comprising Dicranopteris and Gleichenella. However, it is also important to note that the distinction between hairs and scales is far from clear in some gleichenioid ferns with transition forms found rather frequently (Kramer 1990). Most importantly, Rouxopteris (Gleichenia) boryi possesses also hairs instead of scales.
Many Cretaceous gleichenioid fern fossils have not been considered in a phylogenetic framework, which is likely the most promising approach to resolve the currently ambiguous taxonomy of these fossils (Nagalingum and Cantrill 2006). This kind of study may also allow to rediscover the evidence needed to test the hypothesis of a Cretaceous radiation of the crown group of Gleicheniaceae including evidence for the establishment of the Rouxopteris (Gleichenia) boryi lineage in the Cretaceous. In turn, the integration of fossil evidence may also provide the opportunity to test the biogeographic hypothesis suggested by the discovery of Rouxopteris (Gleichenia) boryi as an independent lineage dating back to the Mesozoic. This species occurs today exclusively in Madagascar and Reunion Island. This pattern appears to be consistent with the hypothesis of an origin of this taxon before or in conjunction with the separation of Madagascar from other continents belonging to southern Gondwana. This is notable given the pattern found in the Gleichenia-Stromatopteris clade, which shows a distribution consistent with the southern Gondwana origin hypothesis. In this context, studies on the relationships of the East African Gl. elongata and the Tasmanian Gl. abscida may provide important insights (Verdcourt 2000). Based on morphology alone, it is impossible to decide whether these taxa are closely related to Gl. boryi or to the Gleichenia clade including Gl. polypodioides. A more comprehensive taxon sampling combined with the integration of the known fossil records of these ferns will likely provide the most reliable framework for reconstructing the biogeographic history of gleichenioid ferns. This is especially important because the early divergence of the Gleicheniaceae crown group may be shaped by continental movements following the breakup of the Laurasia and Gondwana continents during the late Mesozoic and early Cenozoic.
Perspectives
The phylogenetic history of Gleicheniaceae has not been studied in detail despite this fern family include many widespread and ecological important species. This study demonstrates that the crown group of this fern lineage diversified during the Cretaceous arguably in the shadow of angiosperms. Two monotypic genera, namely the New Caledonian endemic Stromatopteris and the here described Rouxopteris, are relicts from this early diversification that have survived in remote areas. The newly described genus may have persisted in Madagascar since the breakup of southern Gondwana which makes this taxon arguably unique in the malagasy flora and of special interest to programs aiming to conserve the natural heritage of Madagascar and the Mascarene Islands.
Taxonomic treatment
Our phylogenetic analyses recovered Rouxopteris (Gleichenia) boryi as separated from Gleichenia. This result is consistent with the distinct leaf morphology, which was already pointed out by Kunze (1844). Phylogenetically, the species is not closely related to any species belonging to the currently recognized genera Dicranopteris, Diplopterygium, Gleichenia, Gleichenella, Sticherus, and Stromatopteris. It is very unlikely that additional DNA sequence markers will overturn this result because the separation was found consistently in all analyses carried out. Thus, we introduce a new genus to reflect the separation of this species from other extant gleichenioid ferns. No existing generic name can be linked to the Rouxopteris (Gleichenia) boryi clade. Both previously used generic names, Gleichenia and Gleicheniastrum C.Presl (Presl 1847) are typified with species nested in the Gleichenia clade. Therefore, a new generic name is introduced here.
Rouxopteris H.M. Liu, gen. nov.—TYPE: Rouxopteris boryi (Kunze) H.M. Liu.
Description: Terrestrial ferns. Rhizome long creeping, with a protostelic arrangement of the metaxylem, covered with long deciduous, brown, uniseriate, simple or branched hairs; leaves distant; lamina 1-pinnate-pinnatisect to 2-pinnate, without apical dormant buds; ultimate segments sessils, adnate, deeply lobed; venation free, lateral veins of each ultimate segment 2-bifurcate; medial exindusiate superficial sori, containing 4–9 subsessile sporangia with a gleichenioid type of annulus; spores trilete.
Distribution: Madagascar and Mascarene Islands (La Réunion). Further details on the occurrences of this taxon will be given in treatment of the Flora of Madagascar currently compiled by France Rakotondrainibe.
Etymology: The generic name is dedicated to the pteridologist Jacobus Petrus Roux (1954–2013) who made major contributions to the taxonomy of the ferns occurring in the Afromadagascan region. The name translates as fern of Roux.
Species list
Rouxopteris boryi (Kunze) H.M. Liu, comb. nov.
Rouxopteris boryi var.boryi ≡ Gleichenia boryi Kunze, Farnkr. 1.: 162, t. 70, f. 1. 1844. ≡ Gleicheniastrum boryi (Kunze) C.Presl, Abh. Böhm. Ges. Wiss. 5: 338. 1848*.— TYPE: La Réunion, Plaine des Fougeres, P. Lepervanche s.n. (holotype: unknown; isotypes: BM!, P01300183!).
Rouxopteris boryi var.madagascariensis (C.Chr.) Tardieu ≡ Gleichenia boryi var. madagascariensis (C.Chr.) Tardieu, Bull. Mus. Natl. Adansonia sér. 4(1–2): 105. 1982. ≡ Gleichenia madagascariensis C.Chr., Cat. Pl. Mad. Pter.: 64. 1832 (nom. nud.), Dansk Bot. Ark. 7: 173, t. 69. 1932.—TYPE: Madagascar, Mont Tsaratanana, s.d., Perrier de la Bathie 15525 (holotype: BM000785359!; isotype: P02280076!).
Note: Dr. Peter Otto, curator of the Herbarium Universitatis Lipsiensis (LZ) at Leipzig (Germany), confirmed that the specimen cited as the type of Gleichenia boryi is not among the Kunze collections preserved at the institute. The specimen may have lost during the destruction of the herbarium during the Second World War. However, we can not exclude the possibility that some undetected type material may exist in other collections and thus we have not yet assigned any replacement for the original type material.
References
Badré F (2008) Gleicheniaceae. In: Bosser J, Badré F, Guého J (eds) Flore des Mascareignes, La Réunion, Maurice, Rodrigues. Ptéridophytes, familles 1 a 26. IIRD, MSIRI, RBG-Kew, pp 65–70
Baker JG (1901) Diagnoses Africanae XIII. Kew Bull 119–138
Bernhardi JJ (1805) Dritter Versuch einer Anordnung der Farrnkraeuter. Schrader’s Neues J Bot 1:1–50
Bierhorst DW (1968) On the Stromatopteridaceae (fam. Nov.) and on the Psilotaceae. Phytomorphology 18:232–268
Bryant D, Moultan V (2004) Neighbor-Net: an agglomerative method for the construction of phylogenetic networks. Molec Biol Evol 21:255–265. https://doi.org/10.1093/molbev/msh018
Ching RC (1940) On the genus Gleichenia Smith. Sunyatsenia 5:269–289
Ching RC (1978) The Chinese fern families and genera: systematic arrangement and historical origin. Acta Phytotax Sin 16:16–37
Ching RC, Wang CH, Shing GH (1959) Glecheniaceae. In: Ching RC (ed) Flora Reipublicae Popularis Sinicae, vol. 2. Science Press, Beijing, pp 116–132
Chinnock RJ, Bell GH (1998) Gleicheniaceae. In: Flora of Australia 48, Ferns, Gymnopserms and allied Groups. ABRS/CRISRO, Melbourne, pp 148–161
Christenhusz MJM, Zhang XC, Schneider H (2011) A linear sequence of extant families and genera of lycophytes and ferns. Phytotaxa 19:7–54
Christensen C (1906) Index Filicum LV. Apud H, Hagerup, Hafniae
Christensen C (1932a) The Pteridophyta of Madagascar. Dansk Bot Ark 7:1–253
Christensen C (1932b) Filices. In: Perrier de la Bathie H (ed) Catalogue des Plantes des plantes de Madagascar. Imprimerie G. Pitot & Cie, Tananarive, pp 1–72
Chrysler MA (1944) The vascular structure of the leaf of Gleichenia. II. Petiolar bundle. Amer J Bot 31:483–491
Copeland EB (1909) New genera and species of Bornean ferns. Philipp J Sci C, Bot 3:343–351
Copeland EB (1947) Genera Filicum, the genera of ferns. Chronica Botanica, Waltham
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModeltest 2: more models, new heuristics and parallel computing. Nat Meth 9:772
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull Bot Soc Amer 19:11–15
Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUTi and BEAST 1.7. Molec Biol Evol 29:1969–1973. https://doi.org/10.1093/molbev/mss075
Gandolfo M, Nixon K, Crepet W, Ratcliffe G (1997) A new fossil fern assignable to Gleicheniaceae from Late Cretaceous sediments of New Jersey. Amer J Bot 84:483–493. https://doi.org/10.2307/2446025
Gonzales JR, Kessler M (2011) A synopsis of the Neotropical species of Sticherus (Gleicheniaceae), with descriptions of nine new species. Phytotaxa 31:1–54
Grangaud E (2010) Guide des Fougères et plantes alliées des Mascareignes - La Réunion, Maurice et Rodrigues. Biotope, Mèze (collection Parthénope); Muséum national d’ Historie naturelle, Paris
Hennequin S, Kessler M, Lindsay S, Schneider H (2014) Evolutionary patterns in the assembly of fern diversity on the Mascarene Islands. J Biogeogr 41:1651–1663. https://doi.org/10.1111/jbi.12339
Herendeen PS, Skog JE (1998) Gleichenia chaloneri - a new fossil fern from the lower Cretaceous (Albian) of England. Int J Pl Sci 159: 870–879. https://www.journals.uchicago.edu/doi/10.1086/297609
Holttum RE (1957a) Morphology, growth-habit, and classification in the family Gleicheniaceae. Phytomorphology 8:168–184
Holttum RE (1957b) On the taxonomic subdivision of the Gleicheniaceae with descriptions of the new Malaysian species and varieties. Reinwardtia 4:257–280
Holttum RE (1959) Gleicheniaceae. Flora Malesiana Series 2. Pteridophyte 1:1–36
Huson DH, Bryant D (2006) Application of phylogenetic networks in evolutionary studies. Molec Biol Evol 23:254–267. https://doi.org/10.1093/molbev/msj030
Jin XF, Ding BY, Iwatsuki K (2013) Gleicheniaceae. In: Wu ZY, Raven PH, Hong DY (eds) Flora of China, vols. 2–3. Science Press, Beijing; Missouri Botanical Garden Press, St. Louis, pp 110–115
John SH (1942) New combinations in the Gleicheniaceae and in Stephelia (Epacridaceae). Occas Pap Bernice Pauahi Bishop Mus 17:79–84
Kramer KU (1990) Gleicheniaceae. In: Kubitzki K (ed) The Families and Genera of Vascular Plants, vol. 1. Pteridophytes and Gymnosperms. Springer, Berlin, pp 145–152
Kunze G (1844) Farnkraeuter in kolorirten Abbildungen naturgetreu erlaeutert und beschrieben. Schtuhr’s Farnkraeuter Supplement Band 1, Heft 7. Ernst Fleischer, Leipzig
Li CX, Lu SG, Ma JY, Yang Q (2010) Phylogeny and divergence of Gleicheniaceae inferred from three plastid genes. Acta Palaeontol Sin 49:64–72
Lockhart PJ, Steel MA, Hendy MD, Penny D (1994) Recovering evolutionary trees under a more realistic model of sequence evolution. Molec Biol Evol 11:605–612
Maddison WP, Maddison DR (2018) Mesquite: a modular system for evolutionary analysis. Version 3.50. Available at: http://mesquiteproject.org
Mettenius GH (1861) Filices Novae Caledoniae. Ann Sci Nat, Bot 4:55–91
Mildbread J (1934) Neue und seltene Arten aus Ostafrika (Tanangyika-Territ. Mandat) leg. H.J. Schlieben VI. Notizbl Königl Bot Gart Mus Berlin 12:79–109
Nagalingum NS, Cantrill DJ (2006) Early Cretaceous Gleicheniaceae and Matoniaceeae (Gleicheniales) from Alexander Island, Antarctica. Rev Palaeobot Palynol 138:73–93. https://doi.org/10.1016/j.revpalbo.2005.11.001
Nakai T (1950) A new classification of Gleicheniales. Bull Nat Sci Mus 29:1–71
Nakaike T (1975) Enumeratio Pteridophytarum Japonicarum. University Tokyo Press, Tokyo, Filicales
Perrie LR, Bayly MJ, Lehnebach CA, Brownsey PJ (2007) Molecular phylogenetic and molecular dating of the New Zealand Gleicheniaceae. Brittonia 59:129–141
Perrie LR, Shepherd LD, Brownsey PJ (2012) Gleichenia inclusisora, a new and uncommon tangle fern from New Zealand. New Zealand J Bot 50:401–410. https://doi.org/10.1080/0028825X.2012.724015
PPG1 (2016) A community-derived classification for extant lycophytes and ferns. J Syst Evol 54:563–603. https://doi.org/10.1111/jse.12229
Presl CB (1825) Reliquiae Haenkeanae seu descritiones et ícones plantarum quas in America meridionali et boreali, in insulsi Philippinis et Marianis colleget Thaddaeus Haenke, Tomus 1. J.G. Calve, Prague
Presl CB (1836) Tentamen Pteridographiae seu genera filicacearum praisertim junta venarum decursum et distributional exposita. Typis Filiorum Theophili Haase, Prague
Presl CB (1847) Die Gefaessbuendel im Stipes der Farrn. K.K.Hofbuchdruckerei von Gottlieb Haase Söhne, Prague
Presl CB (1851) Epimeliae Botanicae. Abh Königl Böhm Ges Wiss 4:361–624
Pryer KM, Schuettpelz E, Wolf PG, Schneider H, Smith AR, Cranfill R (2004) Phylogeny and evolution of ferns (monilophytes) with a focus on the early leptosporangiate divergences. Amer J Bot 91:1582–1598. https://doi.org/10.3732/ajb.91.10.1582
Pybus OG, Harvey PH (2000) Testing macro-evolutionary models using incomplete molecular phylogenies. Proc Roy Soc London B 267:2267–2272. https://doi.org/10.1098/rspb.2000.1278
Reinward CGC (1825) Filices. In: Hornschuch CF (ed) Sylloge Plantarum Novrarum itemque minus cognitarum: a praestantissimis botanicus adhuc viventibus collecta et a Societate regia botanica Ratisbonensi 2. Typis viduae C.E.Brenk, Regensburg, pp 2–3
Rodway L (1903) Tasmanian Flora. J. Vail, Government Printer, Hobart
Ronquist F, Teslenk M, van der Mark P, Ayres DL, Darling A, Hoehna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Roux JP (2009) Synopsis of the Lycopodiophyta and Pteridophyta of Africa, Madagascar and neighbouring islands. Strelitzia 23:1–296
Schneider H, Schuettpelz E, Pryer KM, Cranfill R, Magallon S, Lupia R (2004) Ferns diversified in the shadow of angiosperms. Nature 428: 553–557. https://www.nature.com/articles/nature02361
Schuettpelz E, Pryer KM (2007) Fern phylogeny inferred from 400 leptosporangiate species and three plastid genes. Taxon 56:1037–1050. https://doi.org/10.2307/25065903
Schuettpelz E, Pryer KM (2009) Evidence for a Cenozoic radiation of ferns in an angiosperm-dominated canopy. Proc Nat Acad Sci USA 106:11200–11205. https://doi.org/10.1073/pnas.0811136106
Shaw SW, Ranker TA (2011) New and improved leaf terminology for Gleicheniaceae. Amer Fern J 101:117–124. https://doi.org/10.1640/0002-8444-101.2.117
Shu JP, Shang H, Jin DM, Wei HJ, Zhou XL, Liu HM, Gu YF, Wang Y, Wang FG, Shen H, Zhang R, Adjie B, Yan YH (2017) Re-establishment of species from synonymies based on DNA barcoding and phylogenetic analysis using Diplopterygium simulans (Gleicheniaceae) as an example. PLoS ONE 12:e0164604. https://doi.org/10.1371/journal.pone.0164604
Skog JJ (2001) Biogeography of Mesozoic leptosporangiate ferns related to extant ferns. Brittonia 53:236–269
Smith JE (1973) Tentamen botanique de filicum generis dorsiferaraum. Mém Acad Roy Sci Turin 5:401–423
Smith AR, Pryer KM, Schuettpelz E, Korall P, Schneider H, Wolf PG (2006) A classification for extant ferns. Taxon 55:705–731. https://doi.org/10.2307/25065646
Smith AR, Pryer KM, Schuettpelz E, Korall P, Schneider H, Wolf PG (2008) Fern classification. In: Haufler CH, Ranker TA (eds) Biology and Evolution of Ferns and Lycophytes. Cambridge University Press, Cambridge, pp 417–465
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. https://doi.org/10.1093/bioinformatics/btl446
Swofford DL (2003) PAUP: phylogenetic analysis using parsimony (and other methods). Version 4.0b10. Sinauer Associates, Sunderland
Tardieu-Blot M-L (1982) Espèce et combinaison nouvelles de fougères des Mascareignes. Bull Mus Natl Hist Nat B sér. 4:103–105
Testo W, Sundue M (2014) A 4000-species dataset provides new insight into the evolution of ferns. Molec Phylogen Evol 105:200–211. https://doi.org/10.1016/j.ympev.2016.09.003
Tryon AF, Lugardon B (1991) The Spore of the Pteridophyta. Springer-Verlag, New York
Tryon RM, Tryon AF (1982) Ferns and allied Plants. Springer-Verlag, New York
Underwood LM (1907) American Ferns VIII. A preliminary review of the North American Gleicheiniaceae. Bull Torrey Bot Club 34:243–262
Verdcourt B (2000) Gleicheniaceae. In: Beentje HJ (ed) Flora of East Africa, Gleicheniaceae. AA Balkema, Rotterdam, pp 1–9
Wallich N (1829) Plantae Asiaticae Rariores or descriptions and figures of a select number of unpublished East Indian Plants 1. Richard Taylor, Printer to the University of London, London
Acknowledgements
Fieldwork in La Réunion Island was supported by the Conservatoire Botanique National de Mascarin, Parc National de La Réunion and the Universite de la Réunion. The study was financially supported by a Marie Curie Fellowship (PIEF-GA-2008-200447), a Darwin Now Award from the British Council (2009) and Recruitment Program of Global Experts (WQ2017530454), all three awarded to SH, and by projects from Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences (2017XTBG-T03, 2017XTBG-F05) and funding from the Scientific Research Program of Sino-Africa Joint Research Center (SAJC201607). We thank Edmond Grangaud and Jean-Maurice Tamon for their kind support during visits to La Réunion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors are not aware of any potential conflicts of interests.
Human and animal rights
A plant-based research project the research did not involve human participants or animal-based experiments. All collections and experiments were carried out with the required permissions and standards.
Additional information
Handling editor: Martin A. Lysak.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
606_2020_1657_MOESM1_ESM.nex
Supplementary material 1 Molecular Dataset used to reconstruct the Phylogenetic Hypothesis: Dataset includes all rbcL seqeunces given as a nexus format (NEX 56 kb)
Information on Electronic Supplementary Material
Information on Electronic Supplementary Material
Online Resource 1. Aligned matrix of rbcL sequences used in the study.
Rights and permissions
About this article
Cite this article
Liu, H., Rakotondrainibe, F., Hennequin, S. et al. The significance of Rouxopteris (Gleicheniaceae, Polypodiopsida): a new genus endemic to the Madagascan region. Plant Syst Evol 306, 30 (2020). https://doi.org/10.1007/s00606-020-01657-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00606-020-01657-9