Abstract
A 3D printed, automated, pressure-driven injection microfluidic system for microchip electrophoresis (µCE) of preterm birth (PTB)-related peptides and proteins has been developed. Functional microvalves were formed, either with a membrane thickness of 5 µm and a layer exposure time of 450 ms or with a membrane thickness of 10 µm and layer exposure times of 300–350 ms. These valves allowed for control of fluid flow in device microchannels during sample injection for µCE separation. Device design and µCE conditions using fluorescently labeled amino acids were optimized. A sample injection time of 0.5 s and a separation voltage of 450 V (460 V/cm) yielded the best separation efficiency and resolution. We demonstrated the first µCE separation with pressure-driven injection in a 3D printed microfluidic device using fluorescently labeled PTB biomarkers and 532 nm laser excitation. Detection limits for two PTB biomarkers, peptide 1 and peptide 2, for an injection time of 1.5 s were 400 pM and 15 nM, respectively, and the linear detection range for peptide 2 was 50–400 nM. This 3D printed microfluidic system holds promise for future integration of on-chip sample preparation processes with µCE, offering promising possibilities for PTB risk assessment.
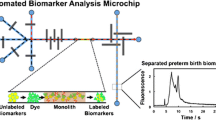
Graphical abstract





Similar content being viewed by others
References
Nielsen JB, Hanson RL, Almughamsi HM, Pang C, Fish TR, Woolley AT (2020) Microfluidics: innovations in materials and their fabrication and functionalization. Anal Chem 92(1):150–168
Urbanska M, Muñoz HE, Shaw BJ, Otto O, Manalis SR, Di Carlo D, Guck J (2020) A comparison of microfluidic methods for high-throughput cell deformability measurements. Nat Methods 17(6):587–593
Wang C, Wang C, Qiu J, Gao J, Liu H, Zhang Y, Han L (2021) Ultrasensitive, high-throughput, and rapid simultaneous detection of SARS-CoV-2 antigens and IgG/IgM antibodies within 10 min through an immunoassay biochip. Microchim Acta 188(8):262
Dai B, Yin C, Wu J, Li W, Zheng L, Lin F, Zhuang S (2021) A flux-adaptable pump-free microfluidics-based self-contained platform for multiplex cancer biomarker detection. Lab Chip 21(1):143–153
Mora MF, Kehl F, Tavares da Costa E, Bramall N, Willis PA (2020) Fully automated microchip electrophoresis analyzer for potential life detection missions. Anal Chem 92(19):12959–12966
Castiaux AD, Selemani MA, Ward MA, Martin RS (2021) Fully 3D printed fluidic devices with integrated valves and pumps for flow injection analysis. Anal Meth 13(42):5017–5024
Sahore V, Sonker M, Nielsen AV, Knob R, Kumar S, Woolley AT (2018) Automated microfluidic devices integrating solid-phase extraction, fluorescent labeling, and microchip electrophoresis for preterm birth biomarker analysis. Anal Bioanal Chem 410(3):933–941
Li F, Guijt RM, Breadmore MC (2016) Nanoporous membranes for microfluidic concentration prior to electrophoretic separation of proteins in urine. Anal Chem 88(16):8257–8263
Mitchell KR, Esene JE, Woolley AT (2022) Advances in multiplex electrical and optical detection of biomarkers using microfluidic devices. Anal Bioanal Chem 414(1):167–180
Qin D, Xia Y, Whitesides GM (2010) Soft lithography for micro-and nanoscale patterning. Nat Protoc 5(3):491
Kim BJ, Meng E (2015) Review of polymer MEMS micromachining. J Micromech Microengin 26(1):013001
Walsh DI III, Kong DS, Murthy SK, Carr PA (2017) Enabling microfluidics: from clean rooms to makerspaces. Trends Biotechnol 35(5):383–392
Morbioli GG, Speller NC, Stockton AM (2020) A practical guide to rapid-prototyping of PDMS-based microfluidic devices: a tutorial. Anal Chim Acta 1135:150–174
Nielsen AV, Beauchamp MJ, Nordin GP, Woolley AT (2020) 3D printed microfluidics. Annu Rev Anal Chem 13:45–65
Waheed S, Cabot JM, Macdonald NP, Lewis T, Guijt RM, Paull B, Breadmore MC (2016) 3D printed microfluidic devices: enablers and barriers. Lab Chip 16(11):1993–2013
Arshavsky-Graham S, Enders A, Ackerman S, Bahnemann J, Segal E (2021) 3D-printed microfluidics integrated with optical nanostructured porous aptasensors for protein detection. Microchim Acta 188(3):67
Balavandy SK, Li F, Macdonald NP, Maya F, Townsend AT, Frederick K, Breadmore MC (2021) Scalable 3D printing method for the manufacture of single-material fluidic devices with integrated filter for point of collection colourimetric analysis. Anal Chim Acta 1151:238101
Chen C, Wang Y, Lockwood SY, Spence DM (2014) 3D-printed fluidic devices enable quantitative evaluation of blood components in modified storage solutions for use in transfusion medicine. Analyst 139(13):3219–3226
Munshi AS, Chen C, Townsend AD, Martin RS (2018) Use of 3D printing and modular microfluidics to integrate cell culture, injections and electrochemical analysis. Anal Meth 10(27):3364–3374
Singh M, Tong Y, Webster K, Cesewski E, Haring AP, Laheri S, Johnson BN (2017) 3D printed conformal microfluidics for isolation and profiling of biomarkers from whole organs. Lab Chip 17(15):2561–2571
Gowers SA, Curto VF, Seneci CA, Wang C, Anastasova S, Vadgama P, Boutelle MG (2015) 3D printed microfluidic device with integrated biosensors for online analysis of subcutaneous human microdialysate. Anal Chem 87(15):7763–7770
Quero RF, da Silveira GD, da Silva JAF, de Jesus DP (2021) Understanding and improving FDM 3D printing to fabricate high-resolution and optically transparent microfluidic devices. Lab Chip 21(19):3715–3729
Castiaux AD, Pinger CW, Hayter EA, Bunn ME, Martin RS, Spence DM (2019) PolyJet 3D-printed enclosed microfluidic channels without photocurable supports. Anal Chem 91(10):6910–6917
Gong H, Woolley AT, Nordin GP (2016) High density 3D printed microfluidic valves, pumps, and multiplexers. Lab Chip 16(13):2450–2458
Gong H, Bickham BP, Woolley AT, Nordin GP (2017) Custom 3D printer and resin for 18 μm × 20 μm microfluidic flow channels. Lab Chip 17(17):2899–2909
Sanchez Noriega JL, Chartrand NA, Valdoz JC, Cribbs CG, Jacobs DA, Poulson D, Nordin GP (2021) Spatially and optically tailored 3D printing for highly miniaturized and integrated microfluidics. Nature Commun 12(1):5509
Gong H, Woolley AT, Nordin GP (2018) 3D printed high density, reversible, chip-to-chip microfluidic interconnects. Lab Chip 18(4):639–647
Caruso G, Musso N, Grasso M, Costantino A, Lazzarino G, Tascedda F, Caraci F (2020) Microfluidics as a novel tool for biological and toxicological assays in drug discovery processes: focus on microchip electrophoresis. Micromachines 11(6):593
Chotteau V (2021) Knowing more from less: miniaturization of ligand-binding assays and electrophoresis as new paradigms for at-line monitoring and control of mammalian cell bioprocesses. Curr Opin Biotechnol 71:55–64
Harrison DJ, Manz A, Fan Z, Luedi H, Widmer HM (1992) Capillary electrophoresis and sample injection systems integrated on a planar glass chip. Anal Chem 64(17):1926–1932
Drevinskas T, Maruška A, Girdauskas V, Dūda G, Gorbatsova J, Kaljurand M (2020) Complete capillary electrophoresis process on a drone: towards a flying micro-lab. Anal Meth 12(41):4977–4986
Mecker LC, Martin RS (2008) Integration of microdialysis sampling and microchip electrophoresis with electrochemical detection. Anal Chem 80(23):9257–9264
Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Black RE (2016) Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 388(10063):3027–3035
Esplin MS, Merrell K, Goldenberg R, Lai Y, Iams JD, Mercer B, Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network (2011) Proteomic identification of serum peptides predicting subsequent spontaneous preterm birth. Am J Obstet Gynecol 204(5) 391-e1
Graves SW, Esplin MS (2011) 80: Validation of predictive preterm birth biomarkers obtained by maternal serum proteomics. Am J Obstet Gynecol 204(1):S46
Anfossi L, Di Nardo F, Cavalera S, Giovannoli C, Baggiani C (2018) Multiplex lateral flow immunoassay: an overview of strategies towards high-throughput point-of-need testing. Biosensors 9(1):2
Hosseini S, Vázquez-Villegas P, Rito-Palomares M, Martinez-Chapa SO (2018) Advantages, disadvantages and modifications of conventional ELISA. In Enzyme-Linked Immunosorbent Assay (ELISA) (pp. 67–115). Springer Singapore.
Beauchamp MJ, Nielsen AV, Gong H, Nordin GP, Woolley AT (2019) 3D printed microfluidic devices for microchip electrophoresis of preterm birth biomarkers. Anal Chem 91(11):7418–7425
Nielsen AV, Nielsen JB, Sonker M, Knob R, Sahore V, Woolley AT (2018) Microchip electrophoresis separation of a panel of preterm birth biomarkers. Electrophoresis 39(18):2300–2307
Almughamsi HM, Howell MK, Parry SR, Esene JE, Nielsen JB, Nordin GP, Woolley AT (2022) Immunoaffinity monoliths for multiplexed extraction of preterm birth biomarkers from human blood serum in 3D printed microfluidic devices. Analyst 147(4):734–743
Bickham AV, Pang C, George BQ, Topham DJ, Nielsen JB, Nordin GP, Woolley AT (2020) 3D printed microfluidic devices for solid-phase extraction and on-chip fluorescent labeling of preterm birth risk biomarkers. Anal Chem 92(18):12322–12329
Sonker M, Parker EK, Nielsen AV, Sahore V, Woolley AT (2018) Electrokinetically operated microfluidic devices for integrated immunoaffinity monolith extraction and electrophoretic separation of preterm birth biomarkers. Analyst 143(1):224–231
Funding
The study was funded by the National Institutes of Health (R01 EB027096).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A.V.B., G.P.N., and A.T.W. all own shares in Acrea 3D, a company that is commercializing 3D printing of microfluidics.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
(MP4 2.78 MB)
(MP4 3.70 MB)
Supplementary file3
(PDF 302 KB)
Rights and permissions
About this article
Cite this article
Esene, J.E., Boaks, M., Bickham, A.V. et al. 3D printed microfluidic device for automated, pressure-driven, valve-injected microchip electrophoresis of preterm birth biomarkers. Microchim Acta 189, 204 (2022). https://doi.org/10.1007/s00604-022-05303-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05303-8