Abstract
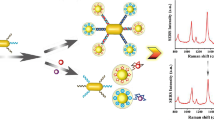
A SERS-based aptasensor for ochratoxin A (OTA) is described. It is making use of Fe3O4@Au magnetic nanoparticles (MGNPs) and of Au@Ag nanoprobes modified with the Raman reporter 5,5-dithiobis-(2-nitrobenzoic acid; DTNB). Au-DTNB@Ag NPs were modified with the OTA aptamer (aptamer-GSNPs) and used as Raman signal probes. The SERS peak of DTNB at 1331 cm−1 was used for quantitative analysis. MGNPs modified with cDNA (cDNA-MGNPs) were used as capture probes and reinforced substrates. When the Au-DTNB@Ag-Fe3O4@Au complexes are formed through oligonucleotide hybridization, the Raman signal intensity of the Raman probe is significantly enhanced. If the OTA concentration in samples increases, more Raman signal probes (aptamer-GSNPs) will dissociate from the cDNA-MGNPs because more OTA aptamer is bound by OTA. This leads to a lower Raman signal after magnetic separation. Under the optimal conditions, the detection limit for OTA is 0.48 pg·mL−1 based on 3σ criterion. This is attributed to the multiple Raman signal enhancement and the good performance of the OTA aptamer. The good recovery and accuracy of the assay was confirmed by evaluating spiked samples of wine and coffee.

Schematic of an aptamer based SERS assay for OTA by integrating Fe3O4@AuNPs (MGNPs) with Au-DTNB@Ag NPs with multiple signal enhancement. Aptamer modified Au-DTNB@Ag NPs are used as Raman probes, and MGNPs modified with cDNA are used as capture probes and reinforced substrates.







Similar content being viewed by others
References
Hwan HT (2015) Recent advances for the detection of ochratoxin A. Toxins (Basel) 7:5276–5300
International Agency for Research on Cancer (IARC) (1993) IARC Monogr Eval Carcinog Risks Hum 56:489–521
Mishra RK, Akhtar H, GaLle C, Georges I, Jean-Louis M (2016) Sensitive quantitation of Ochratoxin a in cocoa beans using differential pulse voltammetry based aptasensor. Food Chem 192:799–804
Liu RJ, Wu H, Lv L, Kang XJ, Cui CB, Feng J, Guo ZJ (2018) Fluorometric aptamer based assay for ochratoxin a based on the use of exonuclease III. Microchim Acta 185:254
Roland A, Bros P, Bouisseau A, Cavelier F, Schneider R (2014) Analysis of ochratoxin a in grapes, musts and wines by LC-MS/MS: first comparison of stable isotope dilution assay and diastereomeric dilution assay methods. Anal Chim Acta 818:39–45
Hu MH, Huang PC, Suo LL, Wu FY (2018) Polydopamine-based molecularly imprinting polymers on magnetic nanoparticles for recognition and enrichment of ochratoxins prior to their determination by HPLC. Microchim Acta 185:300
Radom F, PM J, MP M, Otlewski J, Jeleń F (2013) Aptamers: molecules of great potential. Biotechnol Adv 31:1260–1274
Kong HY, Jonghoe B (2013) Nucleic acid aptamers: new methods for selection, stabilization, and application in biomedical science. Biomol Ther (Seoul) 21:423–434
Wu JJ, Zhu YY, Xue F, Mei ZL, Yao L, Wang X, Zheng L, Liu J, Liu GD, Peng CF, Chen W (2014) Recent trends in SELEX technique and its application to food safety monitoring. Microchim Acta 181:479–491
Sun H, Zu Y (2015) A highlight of recent advances in aptamer technology and its application. Molecules 20:11959–11980
Cruz-Aguado JA, Penner G (2008) Determination of ochratoxin a with a DNA aptamer. J Agric Food Chem 56:10456–10461
Wang C, Dong X, Liu Q, Wang K (2015) Label-free colorimetric aptasensor for sensitive detection of ochratoxin a utilizing hybridization chain reaction. Anal Chim Acta 860:83–88
Yang J, Gao P, Liu Y, Li R, Ma H, Du B, Wei Q (2015) Label-free photoelectrochemical immunosensor for sensitive detection of Ochratoxin a. Biosens Bioelectron 64:13–18
Yu XH, Lin YH, Wang XS, Xu LJ, Wang ZW, Fu FF (2018) Exonuclease-assisted multicolor aptasensor for visual detection of ochratoxin a based on G-quadruplex-hemin DNAzyme-mediated etching of gold nanorod. Microchim Acta 185:259
Li D, Duan HZ, Wang YH, Zhang QM, Cao HR, Deng W, Li DW (2018) On-site preconcentration of pesticide residues in a drop of seawater by using electrokinetic trapping, and their determination by surface-enhanced Raman scattering. Microchim Acta 185:10
Li D, Duan HZ, Ma YD, Deng W (2018) Headspace-sampling paper-based analytical device for colorimetric/surface-enhanced Raman scattering dual sensing of sulfur dioxide in wine. Anal Chem 90:5719–5727
Qu LL, He SH, Wang JJ, Lin ZC, Barry D, Yang GH, Wang P, Zhang P, Li HT (2017) Fluorescence-surface enhanced Raman scattering dual-mode nanosensors to monitor hydroxyl radicals in living cells. Sensors Actuators B Chem 251:934–941
Kneipp J, Kneipp H, Kneipp K (2008) SERS-a single-molecule and nanoscale tool for bioanalytics. Chem Soc Rev 37:1052–1060
Xu LJ, Lei ZC, Li J, Zong C, Yang CJ, Ren B (2015) Label-free surface-enhanced Raman spectroscopy detection of DNA with single-base sensitivity. J Am Chem Soc 137:5149–5154
Zhou Y, Lee C, Zhang J, Zhang P (2013) Engineering versatile SERS-active nanoparticles by embedding reporters between au-core/ag-shell through layer-by-layer deposited polyelectrolytes. J Mater Chem C 1:3695–3699
Song L, Mao K, Zhou X, Hu J (2016) A novel biosensor based on au@ag core-shell nanoparticles for SERS detection of arsenic (III). Talanta 146:285–290
Yu Z, Smith ME, Zhang JN, Zhou Y, Zhang P (2018) Determination of trichloroethylene by using self-referenced SERS and gold-core/silver-shell nanoparticles. Microchim Acta 185(7):330
Feng Y, Wang Y, Wang H, Chen T, Tay YY, Yao L, Yan Q, Li S, Chen H (2012) Engineering “hot” nanoparticles for surface-enhanced Raman scattering by embedding reporter molecules in metal layers. Small 8:246–251
Duan N, Chang B, Zhang H, Wang ZP, Wu SJ (2016) Salmonella typhimurium detection using a surface-enhanced Raman scattering-based aptasensor. Int J Food Microbiol 218:38–43
Kang Y, Hu Y, Ning D (2016) A novel biosensor based on competitive SERS immunoassay and magnetic separation for accurate and sensitive detection of chloramphenicol. Biosens Bioelectron 80:373–377
Wang JJ, Wu X, Wang C, Rong Z, Ding H, Li H, Li S, Shao N, Dong P, Xiao R (2016) Facile synthesis of au-coated magnetic nanoparticles and their application in bacteria detection via a SERS method. ACS Appl Mater Interfaces 8:19958–19967
Zhang H, Ma XY, Liu Y, Duan N, Wu SJ, Wang ZP, Xu BC (2015) Gold nanoparticles enhanced SERS aptasensor for the simultaneous detection of Salmonella typhimurium and Staphylococcus aureus. Biosens Bioelectron 74:872–877
Zhao Y, Yang Y, Luo Y, Yang X, Li M, Song Q (2015) Double detection of mycotoxins based on SERS labels embedded ag@au core-shell nanoparticles. ACS Appl Mater Interfaces 7:21780–21786
Liu J, Che R, Chen H, Zhang F, Xia F, Wu Q, Wang M (2012) Microwave absorption enhancement of multifunctional composite microspheres with spinel Fe3O4 cores and anatase TiO2 shells. Small 8:1214–1221
Wang C, Wang J, Li M, Qu X, Zhang K, Rong Z, Xiao R, Wang S (2016) A rapid SERS method for label-free bacteria detection using polyethylenimine-modified au-coated magnetic microspheres and au@ag nanoparticles. Analyst 141:6226–6238
Ho CC, Zhao K, Lee TY (2014) Quasi-3D gold nanoring cavity arrays with high-density hot-spots for SERS applications via nanosphere lithography. Nanoscale 6:8606–8611
Li W, Zhao X, Yi Z, Glushenkov AM, Kong L (2017) Plasmonic substrates for surface enhanced Raman scattering. Anal Chim Acta 984:19–41
Hu F, Lin H, Zhang Z, Liao F, Shao M, Lifshitz Y, Lee ST (2014) Smart liquid SERS substrates based on Fe3O4/Au nanoparticles with reversibly tunable enhancement factor for practical quantitative detection. Sci Rep 4:7204
Zeng L, Pan Y, Wang S, Wang X, Zhao X, Ren W, Lu G, Wu A (2015) Raman reporter-coupled ag core@au shell nanostars for in vivo improved surface enhanced Raman scattering imaging and near-infrared-triggered photothermal therapy in breast cancers. ACS Appl Mater Interfaces 7:16781–16791
Tschmelak J, Kumpf M, Käppel N, Proll G, Gauglitz G (2006) Total internal reflectance fluorescence (TIRF) biosensor for environmental monitoring of testosterone with commercially available immunochemistry: antibody characterization, assay development and real sample measurements. Talanta 69:343–350
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (21675171, 21277173), the National Instrument Major Project of China (2012YQ3011105), and the Special Fund of State Key Joint Laboratory of Environment Simulation and Pollution Control (17K06ESPCT).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 808 kb)
Rights and permissions
About this article
Cite this article
Song, D., Yang, R., Fang, S. et al. SERS based aptasensor for ochratoxin A by combining Fe3O4@Au magnetic nanoparticles and Au-DTNB@Ag nanoprobes with multiple signal enhancement. Microchim Acta 185, 491 (2018). https://doi.org/10.1007/s00604-018-3020-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3020-2