Abstract
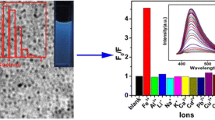
The authors report on the synthesis of boron-doped carbon nanodots (B-C-dots) by a hydrothermal method using ascorbic acid and boric acid as the precursors. The dots have an irregular shape with a typical diameter of 10 nm and display strong fluorescence, with excitation/emission maxima of 350/440 nm. Fluorescence is quenched by Cu(II) and Pb(II) ions due to the formation of nonfluorescent metal complexes between chelating oxygen atoms on the surface of the dots. The findings were used to design a fluorometric method for the determination of the two ions. Linear relationships exist between the drop in fluorescence intensity and the concentrations of Cu(II) and Pb(II) ions in the range from 50 nM to 300 μM and from 25 nM to 250 μM, respectively. The fluorescence of the Cu(II)-quenched B-C-dots is restored in addition of pyrophosphate (PPi) as a result of coordination/chelation interactions between these ions and PPi. This was exploited to develop an assay for PPi that works in the of 25 to 500 μM PPi concentration range.

Boron-doped carbon dots have been synthesized by a hydrothermal method from L-ascorbic acid and boric acid in alkaline solution. The dots are highly selective fluorometric probes for Pb(II), Cu(II) and pyrophosphate ions.






Similar content being viewed by others
References
Zhou J, Zhou H, Tang J, Deng S, Yan F, Li W, Qu M (2017) Carbon dots doped with heteroatoms for fluorescent bioimaging: a review. Microchim Acta 184:343–368
Zu F, Yan F, Bai Z, Xu J, Wang Y, Huang Y, Zhou X (2017) The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim Acta 184(7):1899–1914
Zuo P, Lu X, Sun Z, Guo Y, He H (2016) A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim Acta 183:519–542
Shan X, Chai L, Ma J, Qian Z, Chen J, Feng H (2014) B-doped carbon quantum dots as a sensitive fluorescence probe for hydrogen peroxide and glucose detection. Analyst 139(10):2322–2325
Wang F, Hao Q, Zhang Y, Xu Y, Lei W (2016) Fluorescence quenchometric method for determination of ferric ion using boron-doped carbon dots. Microchim Acta 183(1):273–279
Zhang S, Li J, Zeng M, Xu J, Wang X, Hu W (2014) Polymer nanodots of graphitic carbon nitride as effective fluorescent probes for the detection of Fe3+ and Cu2+ ions. Nano 6:4157–4162
Lee I, Kim S, Kim SN, Jang Y, Jang J (2014) Highly fluorescent amidine/schiff base dual-modified polyacrylonitrile nanoparticles for selective and sensitive detection of copper ions in living cells. ACS Appl Mater Interfaces 6:17151–17156
Qian ZS, Shan XY, Chai LJ, Chen JR, Feng H (2015) A fluorescent nanosensor based on graphene quantum dots-aptamer probe and graphene oxide platform for detection of lead (II) ion. Biosens Bioelectron 68:225–231
Zhao Z, Chen H, Zhang H, Ma L, Wang Z (2017) Polyacrylamide-phytic acid-polydopamine conducting porous hydrogel for rapid detection and removal of copper (II) ions. Biosens Bioelectron 91:306–312
Wang XF, Xiang LP, Wang YS, Xue JH, Zhu YF, Huang YQ, Tang X (2016) A “turn-on” fluorescence assay for lead(II) based on the suppression of the surface energy transfer between acridine orange and gold nanoparticles. Microchim Acta 183(4):1333–1339
Wang C, Cheng H, Sun Y, Xu Z, Lin H, Lin Q, Zhang C (2015) Nanoclusters prepared from a silver/gold alloy as a fluorescent probe for selective and sensitive determination of lead(II). Microchim Acta 182(3–4):695–701
Rao H, Liu W, Lu Z, Wang Y, Ge H, Zou P, Wang X, He H, Zeng X, Wang Y (2016) Silica-coated carbon dots conjugated to CdTe quantum dots: a ratiometric fluorescent probe for copper(II). Microchim Acta 183:581–588
Yan M, Zhu C, Huang Y, Yan J, Chen A (2017) Ultrasensitive detection of lead(II) using a turn-on probe based on the use of an aptamer and a water-soluble fluorescent perylene probe. Microchim Acta 184:2439–2444
Yang R, Guo X, Jia L, Zhang Y (2017) A fluorescent “on-off-on” assay for selective recognition of Cu(II) and glutathione based on modified carbon nanodots, and its application to cellular imaging. Microchim Acta 184:1143–1150
Cleland WW, Hengge AC (2006) Enzymatic mechanisms of phosphate and sulfate transfer. Chem Rev 106:3252–3278
Terkeltaub RA (2001) Inorganic pyrophosphate generation and disposition in pathophysiology. Am J Phys Cell Physiol 281:C1–C11
Hirose M, Abe-Hashimoto J, Ogura K, Tahara H, Ide T, Yoshimura T (1997) A rapid, useful and quantitative method to measure telomerase activity by hybridization protection assay connected with a telomeric repeat amplification protocol. J Cancer Res Clin Oncol 123:337–344
Spangler C, Schaeferling M, Wolfbeis OS (2008) Fluorescent probes for microdetermination of inorganic phosphates and biophosphates. Microchim Acta 161:1–39
Schaeferling M, Wolfbeis OS (2007) Europium tetracycline as a luminescent probe for nucleoside phosphates, and its application to the determination of kinase activity. Chem-Eur J 13:4342–4349
Lu S, Cong R, Zhu S, Zhao X, Liu J, Tse JS (2016) pH-dependent synthesis of novel structure-controllable polymer-carbon nanoDots with high acidophilic luminescence and super carbon dots assembly for white light-emitting diodes. ACS Appl Mater Interfaces 8:4062–4068
Qian R, Ding L, Bao L, He S, Ju H (2012) In situ electrochemical assay of cell surface sialic acids featuring highly efficient chemoselective recognition and a dual-functionalized nanohorn probe. Chem Commun 48:3848–3850
Wang ZX, Ding SN (2014) One-pot green synthesis of high quantum yield oxygen-doped, nitrogen-rich, photoluminescent polymer carbon nanoribbons as an effective fluorescent sensing platform for sensitive and selective detection of silver(I) and mercury(II) ions. Anal Chem 86:7436–7445
Sadhanala HK, Nanda KK (2015) Boron and nnitrogen co-doped carbon nanoparticles as photoluminescent probes for selective and sensitive detection of picric acid. J Phys Chem C 119:13138–13143
Xu Y, Wu M, Feng XZ, Yin XB, He XW, Zhang YK (2013) Reduced carbon dots versus oxidized carbon dots: photo- and electrochemiluminescence investigations for selected applications. Chem-Eur J 19:6282–6288
Xu Y, Wu M, Liu Y, Feng XZ, Yin XB, He XW (2013) Nitrogen-doped carbon dots: a facile and general preparation method, photoluminescence investigation, and imaging applications. Chem-Eur J 19:2276–2283
Kramer R (1998) Fluorescent chemosensors for Cu2+ ions: fast, selective, and highly sensitive. Angew Chem Int Ed 37:772–773
Deng J, Yu P, Yang L, Mao L (2013) Competitive coordination of Cu2+ between cysteine and pyrophosphate ion: toward sensitive and selective sensing of pyrophosphate ion in synovial fluid of arthritis patients. Anal Chem 85:2516–2522
Liu Y, Schanze KS (2008) Conjugated polyelectrolyte-based real-time fluorescence assay for alkaline phosphatase with pyrophosphate as substrate. Anal Chem 80:8605–8612
Park J, Kim Y (2012) A colorimetric probe for the selective naked-eye detection of Pb(ii) ions in aqueous media. Analyst 137:3246–3248
Cornard JP, Dangleterre L, Lapouge C (2005) Computational and spectroscopic characterization of the molecular and electronic structure of the Pb(II)-quercetin complex. J Phys Chem A 109:10044–10051
Kim SK, Day RW, Cahoon JF, Kempa TJ, Song KD, Park HG (2012) Tuning light absorption in core/shell silicon nanowire photovoltaic devices through morphological design. Nano Lett 12:4971–4976
Lv F, Feng X, Tang H, Liu L, Yang Q, Wang S (2011) Development of film sensors based on conjugated polymers for copper(II) ion detection. Adv Funct Mater 21:845–850
Wang ZX, Kong FY, Wang W (2017) Near-ultraviolet fluorescent “on-off-on” switching sensors based on nitrogen-enrichment dual-color single-functional polymer carbon nanosheets. Chem-Eur J 23:665–675
Acknowledgements
We greatly appreciate the support of the National Natural Science Foundation of China (21575123, 21675139, 21603184, 21705140) and the Natural Science Foundation of Jiangsu Province (BK20170474), and the Industry-University-Research Cooperative Innovation Foundation of Jiangsu Province (BY2015057-17).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 655 kb)
Rights and permissions
About this article
Cite this article
Wang, ZX., Yu, XH., Li, F. et al. Preparation of boron-doped carbon dots for fluorometric determination of Pb(II), Cu(II) and pyrophosphate ions. Microchim Acta 184, 4775–4783 (2017). https://doi.org/10.1007/s00604-017-2526-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2526-3