Abstract
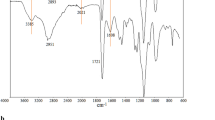
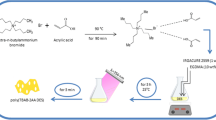
We have designed and synthesized a thermosensitive tri-block copolymer for selective trace extraction of Pb(II) ions from biological and food samples. The polymer was characterized by Fourier transform IR and NMR spectroscopy, and by gel permeation chromatography. The critical aggregation concentration and lower critical solution temperature were determined via fluorescence and UV spectrophotometry, respectively. The effects of solution pH value, amount of copolymer, of the temperature on extraction and on phase separation, and of the matrix on the extraction of Pb(II) were optimized. Pb(II) ions were then quantified by FAAS. The use of this copolymer resulted in excellent figures of merit including a calibration plot extending from 0.5 to 160 μg L−1 (with an R2 of >0.99), a limit of detection (LOD) as low as 90 pg L−1, an extraction efficiency of >98 %, and relative standard deviations of <4 % for eight separate extraction experiments.

In this paper, for the first time an intelligent system using a thermosensitive tri-block copolymer for selective trace removal of Pb(II) in biological and food samples was designed and its determination was carried out by flame atomic absorption spectrometry.




Similar content being viewed by others
References
Jamil M, Zia MS, Qasim M (2010) Contamination of agro-ecosystem and human health hazards from waste water used for irrigation. J Chem Soc Pak 32:370
Khan S, Cao Q, Zheng YM, Huang YZ, Zhu YG (2008) Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing. China Environ Pollut 152:686
Lesmana SO, Febriana N, Soetaredjo FE, Sunarso J, Ismadji S (2009) Studies on potential applications of bio mass for the separation of heavy metals from water and wastewater. Biochem Eng J 44:19
Debelius B, Forja JM, Valls AD, Lubian LM (2009) Toxicity and bioaccumulation of copper and lead in five marine microalgae. Ecotoxicol Environ Saf 72:1503
Jia K, Pan B, Lva L, Zhang Q, Wang X, Pan B, Zhang W (2009) Impregnating titanium phosphate nanoparticles onto a porous cation exchanger for enhanced lead removal from waters. J Colloid Interface Sci 331:453
Wei BG, Yang LS (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94:99–107
Fu FL, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manage 92:407
Behbahani M, Bagheri A, Taghizadeh M, Salarian M, Sadeghi O, Adlnasab L, Jalali K (2013) Synthesis and characterisation of nano structure lead (II) ion-imprinted polymer as a new sorbent for selective extraction and preconcentration of ultra trace amounts of lead ions from vegetables, rice, and fish samples. Food Chem 138:2050
Ryan JA, Scheckel KG, Berti WR, Brown SL, Casteel SW, Chaney RL, Hallfrisch J, Doolan M, Grevatt P, Maddaloni M, Mosby D (2004) Reducing children’s risk from lead in soil. Environ Sci Technol 38:18
Mahaffey KR (1995) Environ. Nutrition and lead: strategies for public health. Health Perspect 103:191
Behbahani M, Najafi M, Amini MM, Sadeghi O, Bagheri A, Salarian M (2013) Dithizone-modified nanoporous fructose as a novel sorbent for solid-phase extraction of ultra-trace levels of heavy metals. Microchim Acta 180:911
Hemmatkhah P, Bidari A, Jafarvand S, Milani Hosseini MR, Assadi Y (2009) Speciation of chromium in water samples using dispersive liquid–liquid microextraction and flame atomic absorption spectrometry. Microchim Acta 166:69
Saçmacı Ş, Kartal Ş (2010) Determination of some trace metal ions in various samples by FAAS after separation/preconcentration by copper(II)-BPHA coprecipitation method. Microchim Acta 170:75
Liang P, Li J, Yang X (2005) Cloud point extraction preconcentration of trace cadmium as 1-Phenyl-3-methyl-4-benzoyl-5-pyrazolone complex and determination by flame atomic absorption spectrometry. Microchim Acta 152:47
Zhu X, Zhao L, Wang B (2006) Speciation analysis of inorganic tin (Sn(II)/Sn(IV)) by graphite furnace atomic absorption spectrometry following ion-exchange separation. Microchim Acta 155:459
Bagheri A, Behbahani M, Amini MM, Sadeghi O, Tootoonchi A, Dahaghin Z (2012) Preconcentration and separation of ultra-trace palladium ion using pyridine-functionalized magnetic nanoparticles. Microchim Acta 178:261
Wang JS, Chiu KH (2009) Metal extraction from solid matrices using a two-surfactant microemulsion in neat supercritical carbon dioxide. Microchim Acta 167:61
Esser-Kahn AP, Iavarone AT, Francis MB (2008) Metallothionein-cross-linked hydrogels for the selective removal of heavy metals from water. J Am Chem Soc 130:15820
Dave N, Chan MY, Huang PJJ, Smith BD, Liu J (2010) Regenerable DNA-functionalized hydrogels for ultrasensitive, instrument-free mercury(II) detection and removal in water. J Am Chem Soc 132:12668
Hazer O, Kartal S (2010) Use of amidoximated hydrogel for removal and recovery of U(VI) ion from water samples. Talanta 82:1974
Xu FJ, Li J, Yuan SJ, Zhang ZX, Kang ET, Neoh KG (2008) Thermo-responsive porous membranes of controllable porous morphology from triblock copolymers of polycaprolactone and Poly(N-isopropylacrylamide) prepared by atom transfer radical polymerization. Biomacromolecules 9:331
Xia Y, Yin X, Burke NAD, Stover HDH (2005) Thermal response of narrow-disperse poly(N-isopropylacrylamide) prepared by atom transfer radical polymerization. Macromolecules 38:5937
Song J, Zhou J, Duan H (2012) Self-assembled plasmonic vesicles of SERS-encoded amphiphilic gold nanoparticles for cancer cell targeting and traceable intracellular drug delivery. J Am Chem Soc 134:13458
Garbern JC, Minami E, Stayton PS, Murry CE (2011) Delivery of basic fibroblast growth factor with a pH-responsive, injectable hydrogel to improve angiogenesis in infarcted myocardium. Biomaterials 32:2407
Garbern JC, Hoffman AS, Stayton PS (2010) Injectable pH- and temperature-responsive poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers for delivery of angiogenic growth factors. Biomacromolecules 11:1833
Sun P, Zhang Y, Shi L, Gan Z (2010) Thermosensitive nanoparticles self-assembled from PCL-b-PEO-b-PNIPAAm triblock copolymers and their potential for controlled drug release. Macromol Biosci 10:621
Jeong B, Kim SW, Bae YH (2002) Thermosensitive sol–gel reversible hydrogels. Adv Drug Deliv Rev 54:37
He C, Kim SW, Lee DS (2008) In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J Control Release 127:189
Chen J, Liu M, Gong H, Huang Y, Chen C (2011) Synthesis and self-assembly of thermoresponsive PEG-b-PNIPAM-b-PCL ABC triblock copolymer through the combination of atom transfer radical polymerization, ring-opening polymerization, and click chemistry. J Phys Chem B 115:14947
Sanna G, Pilo MI, Piu PC, Tapparo A, Seeber R (2000) Determination of heavy metals in honey by anodic stripping voltammetry at microelectrodes. Anal Chim Acta 415:165
Szłyk E, Szydłowska-Czerniak A (2004) Determination of cadmium, lead, and copper in margarines and butters by galvanostatic stripping chronopotentiometry. J Agric Food Chem 52:4064
Hamurcu M, Özcan MM, Dursun N, Gezgin S (2010) Mineral and heavy metal levels of some fruits grown at the roadsides. Food Chem Toxicol 48:1767
Rodushkin I, Axelsson MD (2000) Application of double focusing sector field ICP-MS for multielemental characterization of human hair and nails. Part I. Analytical methodology. Sci Total Environ 250:83
Mortada WI, Sobh MA, El-Defrawy MM, Farahat SE (2002) Reference intervals of cadmium, lead, and mercury in blood, urine, hair, and nails among residents in Mansoura city, Nile Delta, Egypt. Environ Res 90:104
Li CM, Tang YQ, Armes SP, Morris CJ, Rose SF, Lloyd AW, Lewis AL (2005) Synthesis and characterization of biocompatible thermo-responsive gelators based on ABA triblock copolymers. Biomacromolecules 6:994
Surme Y, Narin I, Soylak M, Yuruk H, Dogan M (2007) Cloud point extraction procedure for flame atomic absorption spectrometric determination of lead(II) in sediment and water samples. Michrochim Acta 157:193
Ghanemi K, Nikpour Y, Omidvar O, Maryamabadi A (2011) Sulfur-nanoparticle-based method for separation and preconcentration of some heavy metals in marine samples prior to flame atomic absorption spectrometry determination. Talanta 85:763
Yousefi SR, Shemirani F (2010) Development of a robust ionic liquid-based dispersive liquid–liquid microextraction against high concentration of salt for preconcentration of trace metals in saline aqueous samples: application to the determination of Pb and Cd. Anal Chim Acta 669:25
Acknowledgments
We gratefully acknowledge financial support from the Research Council of Shahid Beheshti University.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 619 kb)
Rights and permissions
About this article
Cite this article
Behbahani, M., Abandansari, H.S., Salarian, M. et al. Synthesis and application of a thermosensitive tri-block copolymer as an efficient sample treatment technique for preconcentration and ultra-trace detection of lead ions. Microchim Acta 181, 129–137 (2014). https://doi.org/10.1007/s00604-013-1079-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-013-1079-3