Abstract
Aims
To analyze retinal vascular plexuses and choriocapillaris by optical coherence tomography angiography (OCT-A) and retinal nerve fiber layer and ganglion cell layer (GCL) by structural optical coherence tomography (OCT) in patients with type 1 diabetes mellitus (T1DM) without diabetic retinopathy (DR).
Methods
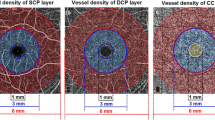
A total of 25 eyes of 25 consecutive T1DM patients without signs of DR were prospectively recruited and compared to 25 healthy subjects (control eyes). All patients underwent OCT-A (CIRRUS HD-OCT model 5000, Carl Zeiss Meditec, Dublin, CA) and structural OCT. Qualitative and quantitative analyses with vessel density were performed on OCT-A images in the superficial capillary plexus (SCP), deep capillary plexus (DCP) and choriocapillaris for all patients.
Results
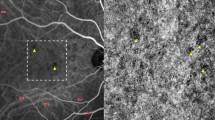
By means of OCT-A, a rarefaction of the perifoveal capillary network in SCP was detected in 7 out of 25 eyes. No significant difference was found in FAZ area of both SCP and DCP comparing diabetic and control groups. By analyzing the DCP, diabetic eyes revealed a significant decreased vessel density compared to control eyes [0.464 ± 0.016 and 0.477 ± 0.014, respectively (p = 0.005)]. Instead, no significant difference was found in the vessel density of all-retina plexus, SCP and choriocapillaris. By RFNL and GCL thickness analysis, no significant differences were disclosed between diabetics and healthy subjects.
Conclusions
We demonstrated the ability of OCT-A to disclose early vascular alterations in patients with T1DM diagnosed as without any signs of DR on the basis of fundus biomicroscopy. Our results also suggest that microvascular changes could precede detectable damage of diabetic neuroretinopathy.


Similar content being viewed by others
References
Prokofyeva E, Zrenner E (2012) Epidemiology of major eye diseases leading to blindness in Europe: a literature review. Ophthalmic Res 47:171–188
Stewart MW (2016) Treatment of diabetic retinopathy: recent advances and unresolved challenges. World J Diabetes 25(7):333–341
Lueder GT, Silverstein J (2005) Screening for retinopathy in the pediatric patient with type 1 diabetes mellitus. Pediatrics 116:270–273
Cho YH, Craig ME, Donaghue KC (2014) Puberty as an accelerator for diabetes complications. Pediatr Diabetes 15:18–26
Fong DS, Aiello L, Gardner TW, American Diabetes Association et al (2003) Diabetic Retinopathy. Diabetes Care 26:226–229
Yanoff M (1969) Ocular pathology of diabetes mellitus. Am J Ophthalmol 67:21–38
Tooke JE (1993) Microvascular haemodynamics in diabetes. Eye 7:227–229
Cade WT (2008) Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther 88:1322–1335
Matsunaga DR, Yi JJ, De Koo LO, Ameri H, Puliafito CA, Kashani AH (2015) Optical coherence tomography angiography of diabetic retinopathy in human subjects. Ophthalmic Surg Lasers Imaging Retina 46:796–805
Kaidonis G, Gillies MC, Abhary S et al (2016) A single-nucleotide polymorphism in the MicroRNA-146a gene is associated with diabetic nephropathy and sight-threatening diabetic retinopathy in Caucasian patients. Acta Diabetol 53:643–650
Mazzeo A, Beltramo E, Iavello A, Carpanetto A, Porta M (2015) Molecular mechanisms of extracellular vesicle-induced vessel destabilization in diabetic retinopathy. Acta Diabetol 52:1113–1119
Rooney D, Lye WK, Tan G et al (2015) Body mass index and retinopathy in Asian populations with diabetes mellitus. Acta Diabetol 52:73–80
Lu J, Hou X, Zhang L et al (2015) Association between body mass index and diabetic retinopathy in Chinese patients with type 2 diabetes. Acta Diabetol 52:701–708
Beltramo E, Lopatina T, Mazzeo A et al (2016) Effects of the neuroprotective drugs somatostatin and brimonidine on retinal cell models of diabetic retinopathy. Acta Diabetol 53:957–964
El-Fayoumi D, Badr Eldine NM, Esmael AF, Ghalwash D, Soliman HM (2016) Retinal nerve fiber layer and ganglion cell complex thicknesses are reduced in children with type 1 diabetes with no evidence of vascular retinopathy. Invest Ophthalmol Vis Sci 57:5355–5360
van Dijk HW, Verbraak FD, Kok PHB et al (2012) Early neurodegeneration in the retina of type 2 diabetic patients. Invest Ophthalmol Vis Sci 53:2715–2719
Vujosevic S, Midena E (2013) Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Müller cells alterations. J Diabetes Res 90:50–58
Tavares Ferreira J, Proença R, Alves M et al (2017) Retina and choroid of diabetic patients without observed retinal vascular changes: a longitudinal study. Am J Ophthalmol. doi:10.1016/j.ajo.2016.12.023
De Benedetto U, Sacconi R, Pierro L, Lattanzio R, Bandello F (2015) Optical coherence tomographic hyperreflective foci in early stages of diabetic retinopathy. Retina 35:449–453
Bandello F, Corbelli E, Carnevali A, Pierro L, Querques G (2016) Optical coherence tomography angiography of diabetic retinopathy. Dev Ophthalmol 56:107–112
Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH (2016) Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci 57:362–370
Dimitrova G, Chihara E, Takahashi H, Amano H, Okazaki K (2017) Quantitative retinal optical coherence tomography angiography in patients with diabetes without diabetic retinopathy. Invest Ophthalmol Vis Sci 58:190–196
Simonett JM, Scarinci F, Picconi F et al (2017) Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol. doi:10.1111/aos.13404
Picconi F, Parravano M, Ylli D et al (2017) Retinal neurodegeneration in patients with type 1 diabetes mellitus: the role of glycemic variability. Acta Diabetol. doi:10.1007/s00592-017-0971-4
Wang RK, Jacques S, Ma Z, Hurst S, Hanson S, Gruber A (2007) Three dimensional optical angiography. Opt Express 15:4083–4097
Wang RK (2010) Optical microangiography: a label free 3d imaging technology to visualize and quantify blood circulations within tissue beds in vivo. IEEE J Sel Top Quantum Electron 16:545–554
An L, Wang RK (2008) In vivo volumetric imaging of vascular perfusion within human retina and choroids with optical micro-angiography. Opt Express 16:11438–11452
Samara WA, Say EA, Khoo CT et al (2015) Correlation of foveal avascular zone size with foveal morphology in normal eyes using optical coherence tomography angiography. Retina 35:2188–2195
Shahlaee A, Pefkianaki M, Hsu J, Ho AC (2016) Measurement of foveal avascular zone dimensions and its reliability in healthy eyes using optical coherence tomography angiography. Am J Ophthalmol 161(50–55):e51
Chidambara L, Gadde SG, Yadav NK et al (2016) Characteristics and quantification of vascular changes in macular telangiectasia type 2 on optical coherence tomography angiography. Br J Ophthalmol. doi:10.1136/bjophthalmol-2015-307941
Battaglia Parodi M, Cicinelli MV, Rabiolo A, Pierro L, Bolognesi G, Bandello F (2016) Vascular abnormalities in patients with Stargardt disease assessed with optical coherence tomography angiography. Br J Ophthalmol. doi:10.1136/bjophthalmol-2016-308869
Scarinci F, Nesper PL, Fawzi AA (2016) Deep retinal capillary nonperfusion is associated with photoreceptor disruption in diabetic macular ischemia. Am J Ophthalmol 168:129–138
Couturier A, Mané V, Bonnin S et al (2015) Capillary plexus anomalies in diabetic retinopathy on optical coherence tomography angiography. Retina 35:2384–2391
de Carlo TE, Chin AT, Bonini Filho MA et al (2015) Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina 35:2364–2370
Freiberg FJ, Pfau M, Wons J, Wirth MA, Becker MD, Michels S (2016) Optical coherence tomography angiography of the foveal avascular zone in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 254:1051–1058
Arend O, Wolf S, Jung F et al (1991) Retinal microcirculation in patients with diabetes mellitus: dynamic and morphological analysis of perifoveal capillary network. Br J Ophthalmol 75:514–518
Sander B, Larsen M, Engler C, Lund-Andersen H, Parving HH (1994) Early changes in diabetic retinopathy: capillary loss and blood-retina barrier permeability in relation to metabolic control. Acta Ophthalmol (Copenh) 72:553–559
La Spina C, Carnevali A, Marchese A, Querques G, Bandello F (2016) Reproducibility and reliability of optical coherence tomography angiography for foveal avascular zone evaluation and measurement in different settings. Retina. doi:10.1097/IAE.0000000000001426
Reis A, Mateus C, Melo P, Figueira J, Cunha-Vaz J, Castelo-Branco M (2014) Neuroretinal dysfunction with intact blood-retinal barrier and absent vasculopathy in diabetes type 1. Diabetes 63:3926–3937
Yoshida A, Kojima M, Ogasawara H, Ishiko S (1991) Oscillatory potentials and permeability of the blood-retinal barrier in noninsulin-dependent diabetic patients without retinopathy. Ophthalmology 98:1266–1271
Spaide RF, Fujimoto JG, Waheed NK (2015) Image artifacts in optical coherence tomography angiography. Retina 35:2163–2180
Rabiolo A, Carnevali A, Bandello F, Querques G (2016) Optical coherence tomography angiography: Evolution or revolution? expert. Rev of Ophthalmol 11:243–245
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Financial disclosures
Adriano Carnevali, Riccardo Sacconi, Eleonora Corbelli, Lea Querques, Gianpaolo Zerbini, Vincenzo Scorcia, Francesco Bandello, Giuseppe Querques: none.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Human and animal rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Managed by Antonio Secchi.
Rights and permissions
About this article
Cite this article
Carnevali, A., Sacconi, R., Corbelli, E. et al. Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol 54, 695–702 (2017). https://doi.org/10.1007/s00592-017-0996-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-017-0996-8