Abstract
Aims
Dioxin or dioxin-like compounds are ligands of the aryl hydrocarbon receptor (AhR), which is a ligand-activated nuclear transcription factor. There are limited studies about the association of serum AhR ligand activities and T2DM. Our objective was to investigate the association of serum AhR ligand activities with T2DM and its related metabolic parameters.
Methods
This case–control study involved 83 subjects with T2DM as well as age-, sex-, and body mass index (BMI)-matched subjects with impaired glucose tolerance (IGT, n = 130) and normal glucose tolerance (NGT, n = 83). Serum AhR ligand activities were measured using a cell-based AhR ligand assay and standardized as 2,3,7,8-tetrachlorodibenzo-p-dioxin equivalents (TCDDeq, pmol/l).
Results
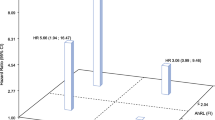
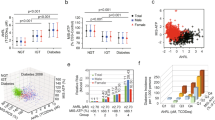
The T2DM group had the highest AhR ligand activities compared to the IGT and NGT groups [median (interquartile range), 68.1 (53.1, 81.5), 60.2 (45.8, 75.1), and 53.3 (46.1, 63.7) pmol/l, respectively; P = 0.003]. In the multivariate analysis, the log2-transformed TCDDeq levels were significantly associated with the risk of T2DM after adjusting for age, sex, and BMI (odds ratio 2.26, 95 % confidence interval 1.34–3.82; P = 0.002). In nondiabetic subjects, serum AhR ligand activities showed a positive correlation with fasting glucose and insulin concentrations and the homeostasis model assessment of insulin resistance, but showed a negative correlation with adiponectin concentrations.
Conclusions
Serum AhR ligand activities were higher in the T2DM group and were correlated with the parameters of insulin resistance. Further investigation is required to elucidate the causal relationship between AhR ligand activity and T2DM.
Similar content being viewed by others
References
Schecter A, Birnbaum L, Ryan JJ, Constable JD (2006) Dioxins: an overview. Environ Res 101(3):419–428
Qing Li Q, Loganath A, Seng Chong Y, Tan J, Philip Obbard J (2006) Persistent organic pollutants and adverse health effects in humans. J Toxicol Environ Health A 69(21):1987–2005
Vasseur P, Cossu-Leguille C (2006) Linking molecular interactions to consequent effects of persistent organic pollutants (POPs) upon populations. Chemosphere 62(7):1033–1042
Gilpin RK, Wagel DJ, Solch JG (2003) Production, Distribution, and Fate of Polychlorinated Dibenzo-p-Dioxins, Dibenzofurans, and Related Organohalogens in the Environment. In: Schecter A, Gasiewicz TA (eds) Dioxins and health, 2nd edn. Wiley, Hoboken, pp 89–136
Mandal PK (2005) Dioxin: a review of its environmental effects and its aryl hydrocarbon receptor biology. J Comp Physiol B 175(4):221–230
Poland A, Knutson JC (1982) 2,3,7,8-tetrachlorodibenzo-thorn-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol 22(1):517–554
Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ (1996) Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol 140(1):173–179
Van den Berg M, Birnbaum L, Bosveld ATC, Brunström B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW, Kubiak T, Larsen JC, Van Leeuwen FX, Liem AK, Nolt C, Peterson RE, Poellinger L, Safe S, Schrenk D, Tillitt D, Tysklind M, Younes M, Warn F, Zacharewski T (1998) Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect 106(12):775–792
Longnecker MP, Michalek JE (2000) Serum dioxin level in relation to diabetes mellitus among air force veterans with background levels of exposure. Epidemiology 11(1):44–48
Fierens S, Mairesse H, J-f Heilier, De Burbure C, J-f Focant, Eppe G, De Pauw E, Bernard A (2003) Dioxin/polychlorinated biphenyl body burden, diabetes and endometriosis: findings in a population-based study in Belgium. Biomarkers 8(6):529–534
Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker BA, Jacobs DR Jr (2006) A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes results from the National Health and Examination Survey 1999–2002. Diabetes Care 29(7):1638–1644
Cox S, Niskar AS, Narayan KV, Marcus M (2007) Prevalence of self-reported diabetes and exposure to organochlorine pesticides among Mexican Americans: hispanic health and nutrition examination survey, 1982–1984. Environ Health Perspect 115:1747–1752
Wang SL, Tsai PC, Yang CY, Guo YL (2008) Increased risk of diabetes and polychlorinated biphenyls and dioxins: a 24-year follow-up study of the Yucheng cohort. Diabetes Care 31(8):1574–1579
Ukropec J, Radikova Z, Huckova M, Koska J, Kocan A, Sebokova E, Drobná B, Trnovec T, Susienkova K, Labudova V, Gasperíková D, Langer P, Klimes I (2010) High prevalence of prediabetes and diabetes in a population exposed to high levels of an organochlorine cocktail. Diabetologia 53(5):899–906
Airaksinen R, Rantakokko P, Eriksson JG, Blomstedt P, Kajantie E, Kiviranta H (2011) Association between type 2 diabetes and exposure to persistent organic pollutants. Diabetes Care 34(9):1972–1979
Taylor KW, Novak RF, Anderson HA, Birnbaum LS, Blystone C, DeVito M, Jacobs D, Köhrle J, Lee D-H, Rylander L, Rignell-Hydbom A, Tornero-Velez R, Turyk ME, Boyles AL, Thayer KA, Lind L (2013) Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a national toxicology program workshop review. Environ Health Perspect 121(7):774–783
Lee DH, Steffes MW, Sjödin A, Jones RS, Needham LL, Jacobs DR Jr (2010) Low dose of some persistent organic pollutants predicts type 2 diabetes: a nested case–control study. Environ Health Perspect 118(9):1235–1242
Lee HK (2011) Mitochondrial dysfunction and insulin resistance: the contribution of dioxin-like substances. Diabetes Metab J 35(3):207–215
Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP (2012) Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33(3):378–455
Barouki R, Coumoul X, Fernandez-Salguero PM (2007) The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett 581(19):3608–3615
Park WH, Jun DW, Kim JT, Jeong JH, Park H, Chang YS, Park KS, Lee HK, Pak YK (2013) Novel cell-based assay reveals associations of circulating serum AhR-ligands with metabolic syndrome and mitochondrial dysfunction. Biofactors 39(4):494–504
American Diabetes Association (2013) Standards of medical care in diabetes, 2013. Diabetes Care 36:S11–S66
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
Lim J-e, Jee SH (2014) Association between serum levels of adiponectin and polychlorinated biphenyls in Korean men and women. Endocrine. doi:10.1007/s12020-014-0231-0 [Epub ahead of print]
Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA (2001) Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86(5):1930–1935
Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8(11):1288–1295
Porta M (2006) Persistent organic pollutants and the burden of diabetes. The Lancet 368(9535):558–559
Enan E, Matsumura F (1994) 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)-induced changes in glucose transporting activity in guinea pigs, mice, and rats in vivo and in vitro. J Biochem Toxicol 9(2):97–106
Kurita H, Yoshioka W, Nishimura N, Kubota N, Kadowaki T, Tohyama C (2009) Aryl hydrocarbon receptor-mediated effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on glucose-stimulated insulin secretion in mice. J Appl Toxicol 29(8):689–694
Sato S, Shirakawa H, Tomita S, Ohsaki Y, Haketa K, Tooi O, Santo N, Tohkin M, Furukawa Y, Gonzalez FJ, Komai M (2008) Low-dose dioxins alter gene expression related to cholesterol biosynthesis, lipogenesis, and glucose metabolism through the aryl hydrocarbon receptor-mediated pathway in mouse liver. Toxicol Appl Pharmacol 229(1):10–19
Wang C, Xu C-X, Krager SL, Bottum KM, Liao D-F, Tischkau SA (2011) Aryl hydrocarbon receptor deficiency enhances insulin sensitivity and reduces PPAR-α pathway activity in mice. Environ Health Perspect 119(12):1739–1744
Patterson DG Jr, Hampton L, Lapeza CR Jr, Belser WT, Green V, Alexander L, Needham LL (1987) High-resolution gas chromatographic/high-resolution mass spectrometric analysis of human serum on a whole-weight and lipid basis for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Anal Chem 59(15):2000–2005
Ziccardi MH, Gardner IA, Denison MS (2000) Development and modification of a recombinant cell bioassay to directly detect halogenated and polycyclic aromatic hydrocarbons in serum. Toxicol Sci 54(1):183–193
Kim JT, Kim SS, Jun DW, Hwang YH, Park WH, Pak YK, Lee HK (2013) Serum arylhydrocarbon receptor transactivating activity is elevated in type 2 diabetic patients with diabetic nephropathy. J Diabetes Investig 4(5):483–491
Acknowledgments
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Education, Science and Technology; NRF-2006-2005410) and partly by the NRF grant funded by the Korea government (Ministry of Science, ICT, and Future Planning; NRF 2008-0061888).
Conflict of interest
Eun Roh, Soo Heon Kwak, Hye Seung Jung, Young Min Cho, Youngmi Kim Pak, Kyong Soo Park, Seong Yeon Kim, Hong Kyu Lee declare that they have no conflict of interest to disclose.
Ethical standard
Ethics approval was obtained from the institutional review board of the Seoul National University Hospital in Seoul, Korea.
Human and animal rights disclosure
The study was approved by the Seoul National University Hospital Institutional Review Board and therefore performed in accordance with the ethical standards of the Helsinki Declaration of 1975, as revised in 2013.
Informed consent disclosure
All study subjects provided informed consent.
Author information
Authors and Affiliations
Corresponding author
Additional information
Managed by Massimo Federici.
Eun Roh and Soo Heon Kwak have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roh, E., Kwak, S.H., Jung, H.S. et al. Serum aryl hydrocarbon receptor ligand activity is associated with insulin resistance and resulting type 2 diabetes. Acta Diabetol 52, 489–495 (2015). https://doi.org/10.1007/s00592-014-0674-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-014-0674-z