Abstract
Aims
Dietary fats have been shown to promote the translocation of bacterial endotoxins from the gut into circulation, which may induce systemic inflammation and modulate the inflammatory response of circulating immune cells. The aim of this study was to determine the effect of the postprandial milieu on inflammation and the inflammatory response of antigen presenting cells in the context of type 1 diabetes (T1D).
Materials and methods
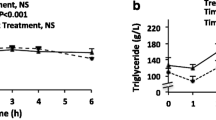
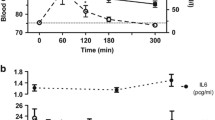
Eleven patients with T1D and eleven nondiabetic controls were recruited as part of the FinnDiane study and given two fatty meals during 1 day. Cytokine responses in monocytes and myeloid dendritic cells (mDCs) as well as serum lipopolysaccharide activity levels, triglyceride concentrations and cytokine concentrations were measured from fasting and postprandial blood samples.
Results
Postprandially, patients with T1D and controls showed significant increases in eight inflammatory cytokines (IL-6, TNF-α, IL-1β, IFN-α, IL-10, IFN-γ, IL-12 and MIP-1β) without concomitant increase in serum LPS activity. Serum cytokine production was similar in both groups. No postprandial change was seen in the IL-6, TNF-α or IL-1β production of mDCs or monocytes. At fasting, diabetic mDCs exhibited higher LPS-induced IL-6 and IL-1β production than controls.
Conclusions
Acute high-fat meals increase circulating cytokines but have no effect on serum lipopolysaccharide activity levels or cytokine production in circulating mDCs or monocytes. Our results suggest that postprandial increase in serum cytokine levels is neither mediated by circulating endotoxins nor the activation of circulating innate cells. The production of high-fat meal-induced inflammatory markers is most likely regulated at the tissue level.


Similar content being viewed by others
References
Cani PD, Delzenne NM (2009) Interplay between obesity and associated metabolic disorders: new insights into the gut microbiota. Curr Opin Pharmacol 9(6):737–743
Mehta NN, McGillicuddy FC, Anderson PD, Hinkle CC, Shah R, Pruscino L, Tabita-Martinez J, Sellers KF, Rickels MR, Reilly MP (2009) Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes 59(1):172–181
Laugerette F, Vors C, Géloën A, Chauvin MA, Soulage C, Lambert-Porcheron S, Peretti N, Alligier M, Burcelin R, Laville M (2011) Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. J Nutr Biochem 22(1):53–59
Vaarala O, Atkinson MA, Neu J (2008) The “Perfect storm” for type 1 diabetes the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes 57(10):2555–2562
Weatherill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH (2005) Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol 174(9):5390–5397
Huang H, Liu T, Rose JL, Stevens RL, Hoyt DG (2007) Sensitivity of mice to lipopolysaccharide is increased by a high saturated fat and cholesterol diet. J Inflamm (Lond) 4:22
Erridge C, Attina T, Spickett CM, Webb DJ (2007) A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr 86(5):1286–1292
Alipour A, van Oostrom AJ, Izraeljan A, Verseyden C, Collins JM, Frayn KN, Plokker TWM, Elte JWF, Cabezas MC (2008) Leukocyte activation by triglyceride-rich lipoproteins. Arterioscler Thromb Vasc Biol 28(4):792–797
Van Oostrom A, Sijmonsma T, Rabelink T, Van Asbeck B, Cabezas MC (2003) Postprandial leukocyte increase in healthy subjects. Metab Clin Exp 52(2):199–202
Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH (2003) Reciprocal modulation of toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem 278(39):37041–37051
Ye J, Keller JN (2010) Regulation of energy metabolism by inflammation: a feedback response in obesity and calorie restriction. Aging (Albany NY) 2(6):361
Zhang G, Ghosh S (2000) Molecular mechanisms of NF-κB activation induced by bacterial lipopolysaccharide through toll-like receptors. J Endotoxin Res 6(6):453–457
Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I (2008) Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab 93(2):578–583
Meyers A, Shah R, Gottlieb P, Zipris D (2010) Altered toll-like receptor signaling pathways in human type 1 diabetes. J Mol Med 88(12):1221–1231
Nymark M, Pussinen PJ, Tuomainen AM, Forsblom C, Groop PH, Lehto M, FinnDiane Study Group (2009) Serum lipopolysaccharide activity is associated with the progression of kidney disease in finnish patients with type 1 diabetes. Diabetes Care 32(9):1689–1693
Lassenius MI, Pietilainen KH, Kaartinen K, Pussinen PJ, Syrjanen J, Forsblom C, Porsti I, Rissanen A, Kaprio J, Mustonen J et al (2011) Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care 34(8):1809–1815
Pussinen PJ, Havulinna AS, Lehto M, Sundvall J, Salomaa V (2011) Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care 34(2):392–397
Saraheimo M, Teppo AM, Forsblom C, Fagerudd J, Groop PH (2003) Diabetic nephropathy is associated with low-grade inflammation in type 1 diabetic patients. Diabetologia 46(10):1402–1407
Laugerette F, Vors C, Peretti N, Michalski M (2011) Complex links between dietary lipids, endogenous endotoxins and metabolic inflammation. Biochimie 93(1):39–45
Nieminen JK, Vakkila J, Salo HM, Ekström N, Härkönen T, Ilonen J, Knip M, Vaarala O (2012) Altered phenotype of peripheral blood dendritic cells in pediatric type 1 diabetes. Diabetes Care 35(11):2303–2310
Alkanani AK, Rewers M, Dong F, Waugh K, Gottlieb PA, Zipris D (2012) Dysregulated toll-like Receptor-Induced interleukin-1β and interleukin-6 responses in subjects at risk for the development of type 1 diabetes. Diabetes 61(10):2525–2533
Hyson DA, Paglieroni TG, Wun T, Rutledge JC (2002) Postprandial lipemia is associated with platelet and monocyte activation and increased monocyte cytokine expression in normolipemic men. Clin Appl Thromb Hemost 8(2):147–155
Pott GB, Chan ED, Dinarello CA, Shapiro L (2009) Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J Leukoc Biol 85(5):886–895
Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R (2008) Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci 105(26):9047–9052
Lassenius MIS, Mäkinen V-P, Fogarty CL, Peräneva L, Jauhiainen M, Pussinen P, Taskinen M-R, Kirveskari J, Vaarala O, Nieminen JK, Hörkkö S, Kangas AJ, Soininen P, Ala-Korpela M, Gordin D, Ahola AJ, Forsblom C, Groop P-H, Lehto M (2014) Patients with type 1 diabetes show signs of vascular dysfunction in response to multiple high-fat meals. Nutr Metab 11:28
Kelly CJ, Colgan SP, Frank DN (2012) Of microbes and meals the health consequences of dietary endotoxemia. Nutr Clin Pract 27(2):215–225
Kuwana T, Rosalki S (1990) Intestinal variant alkaline phosphatase in plasma in disease. Clin Chem 36(11):1918–1921
Honkanen J, Nieminen JK, Gao R, Luopajarvi K, Salo HM, Ilonen J, Knip M, Otonkoski T, Vaarala O (2010) IL-17 immunity in human type 1 diabetes. J Immunol 185(3):1959–1967
Ferraro A, Socci C, Stabilini A, Valle A, Monti P, Piemonti L, Nano R, Olek S, Maffi P, Scavini M (2011) Expansion of Th17 cells and functional defects in T regulatory cells are key features of the pancreatic lymph nodes in patients with type 1 diabetes. Diabetes 60(11):2903–2913
Bradshaw EM, Raddassi K, Elyaman W, Orban T, Gottlieb PA, Kent SC, Hafler DA (2009) Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J Immunol 183(7):4432–4439
Davila E, Kolls J (2010) A “Toll” for Th17 cell expansion. J Leukoc Biol 88(1):5–7
Mallone R, Mannering S, Brooks-Worrell B, Durinovic-Belló I, Cilio C, Wong F, Schloot N (2011) Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T cell responses: position statement of the T-cell workshop committee of the immunology of diabetes society. Clin Exp Immunol 163(1):33–49
Wurfel MM, Kunitake ST, Lichenstein H, Kane JP, Wright SD (1994) Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J Exp Med 180(3):1025–1035
Herieka M, Erridge C (2013) High-fat meal induced postprandial inflammation. Mol Nutr Food Res 58(1):136–146
Pettersson US, Waldén TB, Carlsson P, Jansson L, Phillipson M (2012) Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One 7(9):e46057
Shono S, Habu Y, Nakashima M, Sato A, Nakashima H, Miyazaki H, Kinoshita M, Tsumatori G, Shinomiya N, Seki S (2011) The immunologic outcome of enhanced function of mouse liver lymphocytes and kupffer cells by high-fat and high-cholesterol diet. Shock 36(5):484–493
Acknowledgments
The study was supported by Folkhälsan Research Foundation (PHG), Wilhelm and Else Stockmann Foundation (PHG, ML, MIL, CF), Academy of Finland (134379 to PHG, 257545 to MJ), Liv och Hälsa Foundation (MIL, PHG), Waldemar von Frenckells stiftelse (MIL), Svenska kulturfonden (MIL), Kyllikki and Uolevi Lehikoinen Foundation (MIL), Finnish Cardiovascular Foundation (SH), Helsinki University Central Hospital Research Foundation (MRT), the Sigrid Juselius Foundation (MRT, PJP, SH), Diabetes Research Foundation (ML) and The Novo Nordisk Foundation (PHG, ML). We acknowledge the nurses A. Sandelin, J. Tuomikangas and T. Soppela at the Folkhälsan Institute of Genetics for their excellent contribution to this work.
Conflict of interest
Christopher L. Fogarty, Janne K. Nieminen, Lina Peräneva, Mariann I. Lassenius, Aila J. Ahola, Marja-Riitta Taskinen, Matti Jauhiainen, Juha Kirveskari, Pirkko Pussinen, Sohvi Hörkkö, Ville-Petteri Mäkinen, Daniel Gordin, Carol Forsblom, Per-Henrik Groop, Outi Vaarala and Markku Lehto declare that they have no conflict of interest.
Statement of ethical disclosure
The study protocol was approved by the local ethical committee (221/13/03/01/2009; Ethics Committee, Department of Medicine, Helsinki University Central Hospital).
Statement of human and animal rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of informed consent
Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Managed by Massimo Porta.
On behalf of the FinnDiane Study Group.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fogarty, C.L., Nieminen, J.K., Peräneva, L. et al. High-fat meals induce systemic cytokine release without evidence of endotoxemia-mediated cytokine production from circulating monocytes or myeloid dendritic cells. Acta Diabetol 52, 315–322 (2015). https://doi.org/10.1007/s00592-014-0641-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-014-0641-8