Abstract
Purpose
Abdominal aortic aneurysms (AAAs) affect the vascular perfusion of the lumbar spine. The treatment of AAAs with endovascular aortic aneurysm repair (EVAR) completely occludes the direct vascular supply to the lumbar spine. We hypothesized that patients with AAA who undergo EVAR show a different pattern of spinal degeneration than individuals without AAA.
Methods
In this retrospective institutional review board-approved study, 100 randomly selected patients with AAA who underwent EVAR with computed tomography (CT) scans between 2005 and 2017 were compared with age- and gender-matched controls without AAA. In addition, long-term follow-up CT images (> 6 months before EVAR, at the time of EVAR, and > 12 months after EVAR) of the patients were analysed to compare the progression of degeneration from before to after EVAR. Degeneration scores, lumbar levels with the most severe degeneration, and lumbar levels with progressive degeneration were analysed in all CT images. Fisher’s exact test, Wilcoxon signed-rank test, and Mann–Whitney U test were performed for statistical analyses.
Results
Compared with the control group (n = 94), the most severe degeneration was more commonly detected in the mid-lumbar area in the patient group (n = 100, p = 0.016), with significantly more endplate erosions being detected in the lumbar spine (p = 0.015). However, EVAR did not result in significant additional acceleration of the degenerative process in the long-term follow-up analysis (n = 51).
Conclusion
AAA is associated with atypical, more cranially located spinal degradation, particularly in the mid-lumbar segments; however, EVAR does not seem to additionally accelerate the degenerative process. This observation underlines the importance of disc and endplate vascularization in the pathomechanism of spinal degeneration.
Level of evidence I
Diagnostic: individual cross-sectional studies with consistently applied reference standard and blinding.
Similar content being viewed by others
Introduction
Low back pain is a leading cause of healthcare costs worldwide [1]. The prevalence of low back pain is between 5 and 65% [1], with degenerative changes being the most important aetiological factor. However, the mechanism of spinal degeneration and its prevention are not yet fully understood. Various aetiological factors for spinal degeneration have been reported in the literature; these include genetics [2], age and sex [3], arteriosclerosis [4, 5], smoking [6], diabetes [7] and high mechanical loading of the spine, such as heavy lifting [8]. Due to its multifactorial nature, the exact pathoanatomical process of spinal degradation is still not fully understood. For instance, whether the degeneration begins within the disc or at the endplates remains unknown. In addition, the number of identified contributors to spinal degeneration remains unclear. For centuries, it was believed that spine degeneration is initiated at the intervertebral disc due to its lack of vascularization and innervation (the inside–out theory). More recently, the endplates [9, 10] are being considered the initial spot of degeneration. However, both theories are possible. In the case of trauma or heavy lifting, disc deterioration and degeneration might begin within the nucleus (inside–out). In contrast, with decreased vascular nutrition of the disc, e.g., in patients with a smoking history or diabetes, the degenerative process may begin from the endplates, which are rich in blood vessels (outside–in) [11].
Usually, four pairs of lumbar arteries arise from the aorta and supply the first four lumbar vertebrae and discs via the endplates, which are well vascularized. The fifth lumbar segment is supplied by more variable vessels, such as a fifth lumbar artery pair and sacral and iliolumbar branches [12,13,14]. An extensive collateral network connects the paravertebral vessels [14].
So far, the vascular supply to the lumbar spine has not been occluded in any experimental study in order to observe the extent of its contribution to the degenerative process. However, such a phenomenon has been found to occur in patients with abdominal aortic aneurysms (AAAs). In AAAs, some lumbar arteries are located within the aneurysm wall; therefore, the vascular supply to the lumbar spine is decreased. AAAs are often treated with endovascular aortic aneurysm repair (EVAR) [15, 16], with the proximal end of the prosthesis being infrarenal or juxtarenal and the distal end being located within the common iliac arteries, thereby resulting in immediate occlusion of the first to fifth lumbar arteries at the origin [17, 18].
We hypothesized that AAA and/or EVAR alters the pattern and speed of lumbar spine degeneration. Therefore, the present retrospective study aimed to assess the degenerative changes in the lumbar spine in patients with AAA in comparison with those in controls without AAA. Over the long-term course, the degenerative changes in patients with AAA were also assessed before and after EVAR.
Materials and methods
The present study received ethical committee approval.
Definition of groups and patient recruitment
Case–control groups
Reports of all patients older than 18 years who underwent EVAR for AAAs between January 2005 and December 2017 were retrospectively reviewed.
In total, 100 randomly selected patients who underwent an abdominal computed tomography (CT) scan at the time of EVAR and did not undergo any lumbar spine surgery were included in the patient group (Fig. 1).
The control group consisted of randomly selected patients, who underwent an abdominal CT scan between 2005 and 2018 while being hospitalized for undergoing orthopaedic surgery and were age and gender matched with the patient group. The exclusion criteria included prior lumbar spine surgery and known AAA.
Age and gender matching were performed to reduce biases.
Long-term follow-up analysis before and after EVAR
All the included patients underwent abdominal CT scans at the following timepoints: > 6 months before EVAR, at the time of EVAR, and > 12 months after EVAR. Patients who underwent lumbar spine surgery were not included in the study. Imaging studies were performed with a similar interval between the time of the preceding imaging (> 6 months prior) and EVAR (time interval 1) and between the time of EVAR and the follow-up CT (time interval 2) for every patient (Fig. 1).
The study design and the definition of the groups are summarized in Fig. 2.
CT protocol
The CT protocol for the patients with AAA consisted of contrast-enhanced thoracoabdominal or abdominal CT scans using a 64- or 128-slice multidetector CT (MDCT) scanner. However, as the inclusion period was from 2005 until 2017, different Siemens scanners were used as follows: the Sensation 64 (11/2004–9/2006), the Definition (09/2006–03/2008), the Definition AS (03/2008–05/2009), and afterwards the Definition Flash dual-source 128-slice scanner. Therefore, the protocols might vary slightly.
The CT images of the control group were acquired axially using a 64-MDCT scanner (Brilliance CT, Philips Healthcare). As with the cases, the inclusion period was more than 10 years, and there may be some differences in scanners and protocols.
For the lumbar spine analysis in both the cases and the controls, reformatted sagittal images with a dedicated bone kernel and 2 mm slice thickness were used. The slice interval was equal to or smaller than the slice thickness.
Image analysis
All study data were collected and managed using the research electronic data capture (REDCap) tools [19]. All images were reviewed using a picture archiving and communication system (PACS) workstation (Agfa-Gevaert, or Merlin Diagnostic Workcenter, Phönix-PACS).
One radiologist, with more than 10 years’ experience in radiology and over 5 years’ dedicated musculoskeletal radiology experience, analysed the CT images to detect the lumbar spine level with the most severe degeneration (Th12/L1, L1/2, L2/3, L3/4, L4/5, and L5/S1); if similar severe degeneration on several lumbar levels was found, more than one level was noted. The reader also graded the degeneration of the lumbar spine at every lumbar level, assessing the following: disc height (1 = normal, 2 = slightly decreased, 3 = moderately decreased, and 4 = severely decreased, according to a modified Pfirrmann Classification [20]); disc protrusion (none, small, or severe); endplate erosion according to a modified endplate score (EPS) (1 = no erosion, 2 = thinned endplates but no erosion, 3 = small focal defects, 4 = erosion up to 25% of the vertebral endplate width, 5 = erosion up to 50% of the vertebral endplate width, and 6 = complete endplate damage [21] -Fig. 3); sclerotic endplate changes according to Modic type 3 changes [22]; presence of spondylophytes (yes/no); and modified segmental degeneration grade (grades 1–3 [10]); see Table 1). The presence and type of lumbosacral transitional vertebrae were documented, as these can be associated with higher up lumbar spine level degeneration [23, 24]. Further, the lumbar spine level and the size and expansion of the AAA were documented and measured.
The modified endplate score that was used to grade endplate erosions on CT images, initially described by Rajasekaran et al. on MRI [21]
In the analysis of the long-term follow-up, the lumbar spine levels that showed degeneration progression in time intervals 1 or 2 were documented (Th12/L1, L1/2, L2/3, L3/4, L4/5, and L5/S1). If an endoleak was present, its type and lumbar level were documented (types 1–4 [25, 26]).
A second reader, a radiology resident (fourth year of training), also graded the lumbar level of the most severe degeneration of the lumbar spine (Th12/L1, L1/2, L2/3, L3/4, L4/5, and L5/S1) and noted the lumbar spine level of degeneration progression in the long-term follow-up CTs, if present.
The same analysis was performed for the control group, except for the long-term degeneration progression analysis.
Statistical analysis
The statistical analysis was performed using SPSS (Version 25, IBM Corporation), PRISM (Version 8, GraphPad software), and MATLAB (2019a, The MathWorks, Inc.). The numeric parameters are reported as medians and ranges, and categorical data are reported as absolute and relative frequencies.
To compare the overall tendency of the distribution of the affected vertebral levels between the controls and the cases (at the time of the EVAR procedure), an exact Mann–Whitney U test was employed.
To compare the degeneration progression over time, from before and after the EVAR (time interval 1 in comparison to time interval 2), a Wilcoxon matched-pairs signed-rank test was used. Fisher’s exact test was used to compare a potential association of degeneration progression with the presence of an endoleak.
The inter-reader agreement was tested using kappa statistics, with a kappa < 0 meaning no agreement, a kappa of 0.41–0.6 moderate agreement, a kappa of 0.61–0.8 substantial agreement, and a kappa > 0.81 almost perfect agreement, according to Landis and Koch [27].
A p value of < 0.05 was considered to be indicative of a statistically significant difference.
Results
Study population and groups:
Case–control group analysis: The eligible study cohort for the case–control analysis comprised 100 patients (Fig. 1).
Long-term follow-up analysis (before and after EVAR analysis): A total of 51 patients fulfilled the inclusion and exclusion criteria out of 682 patients undergoing EVAR for an AAA between 2005 and 2017 (Fig. 1).
Cases (with AAA) vs. controls (without AAA) analysis
The patient demographics of the case group (n = 100) prior to EVAR and the age- and sex-matched control group (without AAA, n = 94) are summarized in Table 2. Six subjects in the control group were excluded secondarily because of an incidentally detected AAA (aorta diameter > 3 cm) during the image analysis.
There was a significant difference in the occurrence of the most severe degeneration at the mid-lumbar level(s) in the cases at the time of EVAR compared to the controls (p = 0.016; Fig. 4).
The endplate erosions and the endplate scores summed over all vertebral levels were higher in the case group at the time of EVAR compared to the control group (p = 0.015 and p = 0.04, respectively; Table 3 and Fig. 5) with more endplate erosions at all lumbar levels (Table 4). In contrast, decreased disc height and protrusion were more often found at a higher lumbar level (Th12/L1-L3/L4) in the case group (Table 4). However, they were not significantly different summed over all vertebral levels (p = 0.691 and p = 0.668, Table 3).
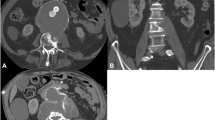
Lumbar spine CTs in a 78-year-old man without a and two patients with abdominal aortic aneurysm (AAA): b 63-year-old man; c: 64-year-old man, showing the most severe degeneration a at L5/S1, b at L5/S1 and L3/4, and c at L3/4 only. Further, note the prominent endplate erosions (arrows) in the patients with AAA b and c
Long-term follow-up analysis for spine degeneration: pre- and post-EVAR comparison
Fifty-one patients (46 male; mean age ± SD = 68.15 ± 8.6) were eligible for long-term analysis of lumbar spine degeneration. The time intervals between prior CT until EVAR (time interval 1) and between EVAR and follow-up CT (time interval 2) were similar (median [range] 1.89 years [0.56–6.13] vs. 1.57 years [0.94–5.76], p = 0.3).
There was no significant difference in lumbar spine degeneration progression frequency before and after EVAR (Table 5).
After EVAR, an endoleak type 1 was documented in one patient and an endoleak type 2 in 14 patients. However, progression of disc degeneration after EVAR was not associated with the presence or absence of an endoleak type 2 (n = 14 vs. n = 37, p = 0.42).
Inter-reader agreement
The inter-reader agreement for the lumbar spine level of the most severe degeneration and the overall levels of degeneration progression of the case group was substantial (kappa 0.75; 95% CI 0.71–0.79) and moderate (kappa 0.60), respectively. Likewise, the inter-reader agreement for the lumbar spine level of the most severe degeneration in the control group was moderate, with a kappa of 0.60 (95% CI 0.52–0.68).
Discussion
In this study, we investigated the natural history of degenerative changes of the lumbar spine in patients with AAA compared to controls without AAA and the degenerative changes before and after EVAR in the same patient to investigate the impact of vascularization on the degeneration process.
This is the first study to report that devascularization (caused by AAA and EVAR) results in an atypical pattern of spinal degeneration when compared to controls without AAA. Further, it is the first report that vascular impairment to the lumbar spine could result in more pronounced endplate erosions. These erosions play a relevant role in low back pain [28] and are potentially the cause of further disc degeneration [10, 28,29,30]. The endplates consist of semi-porous thickened cancellous bone and thin hyaline cartilage type 2 layers, and they are rich in vessels and nerves, supplying the intervertebral disc, where no vessels and nerves are present [9, 28]. Therefore, vascular impairment to the lumbar spine may lead to endplate erosions followed by further degeneration of the disc, as also observed in previous studies [29].
One important finding in this study is that the localization of degenerative changes differs between individuals with and without AAA. It is well known that arteriosclerosis [4] and smoking affect disc degeneration, and Battie et al. reported that smoking affects degeneration of the entire lumbar spine [6]. The severest degeneration in our study was most often observed at the mid-lumbar areas in patients with AAA, while in patients without AAA, the lower lumbar segments were most commonly the severest affected, concordant with previous descriptions [23, 31, 32]. One explanation might be the precise localization of the AAA. In more than 90% of cases, the aneurysm is located below the renal arteries (at lumbar level L2) [17, 33], and its maximal diameter is at lumbar level L3/4. Therefore, the mid-lumbar arteries are likely affected first, as they will be occluded by the aneurysm. In the EVAR procedure, all four pairs of lumbar arteries are over-stented and thereby occluded, which also leads to a diminished blood supply at most in the middle region (the watershed zone).
We observed atypical location of degeneration in the patients with AAA; however, the degeneration grades compared between the patients with AAA and those without were not significantly different, apart from the more commonly observed endplate erosions and a higher endplate score (Table 3).
Although endplate erosions were significantly more present overall in the lumbar spine in the patients with AAA compared to those without, we also observed a relatively high number of endplate erosions also within the control group. This could be due to the age- and sex-matched design, which created a control group with a predominance of elderly males and a higher likelihood of arteriosclerosis, introducing suboptimal vascularization. The aortic dimensions of our control group were therefore also higher than in a normal, healthy, young control group. Also, patients with diabetes and severe arteriosclerosis were not excluded, as it would not be possible to have an absolutely healthy control group at this age. We believe, however, that the control group needed to be age- and sex matched, as we aimed to observe the effect of AAA as a model of relevant local devascularization. Further, our findings are in agreement with Wang et al., who also observed more endplate erosions with greater age, particularly in the lower lumbar region [34]. Regardless of the relatively high number of endplate erosions within the control group, the overall number of erosions was still significantly different between the case and the control groups.
We did not find accelerated lumbar spine degeneration after EVAR of AAA. This finding is consistent with a previous report that sudden occlusion of lumbar arteries due to open surgical repair of AAA did not lead to the development of low back pain or lumbar disc degeneration [35]. A possible explanation could be that the lumbar spine is supplied by a broad collateral system, which might be more pronounced in patients with AAA. The impact of the sudden occlusion of lumbar arteries without a marked collateral system remains unclear. Further, we tested if there was a difference between patients who had an endoleak after EVAR and those who did not. The reason was that in patients with an endoleak type 2, the degeneration progression could be diminished because of a possibly more pronounced collateral blood supply network. However, we did not find a significant difference between the patients with and without a type 2 endoleak. However, this may be because of an insufficient sample size of patients with a type 2 endoleak (n = 14).
Our study has the limitation that the degeneration scoring was performed on CT images. No magnetic resonance images were available for most of the patients who underwent EVAR. However, lumbar spine degeneration can be assessed using CT, as it is routinely performed in daily clinical work [36,37,38]. Because of the retrospective study design, it was not possible to include all the variables reported to be associated with lumbar spine degeneration, such as weight, diabetes, and history of smoking, in the matching process, which led to confounding. However, the relatively high number of degenerative lumbar spine changes in the control group suggests a similar distribution of these variables among the case and control groups. Moreover, the low number of female cases (n = 9/100) leads to a certain bias in our analysis and limits the generalizability of our results.
Conclusions
In conclusion, our study shows that degenerative changes of the lumbar spine are different between individuals with and without AAA: in patients with AAA, the localization of degenerative changes is more at cranial lumbar levels, and more vertebral endplate erosions are detected compared to controls. EVAR does not seem to accelerate degeneration, at least in intermediate long-term follow-up.
Data availability
Authors own work- first author guarantees and has all data.
Code availability
Not applicable.
References
Dagenais S, Caro J, Haldeman S (2008) A systematic review of low back pain cost of illness studies in the United States and internationally. The Spine J 8:8–20. https://doi.org/10.1016/j.spinee.2007.10.005
Battié MC, Videman T, Levälahti E, Gill K, Kaprio J (2008) Genetic and environmental effects on disc degeneration by phenotype and spinal level: a multivariate twin study. Spine 33:2801–2808. https://doi.org/10.1097/BRS.0b013e31818043b7
Miller JA, Schmatz C, Schultz AB (1988) Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine 13:173–178
Kauppila LI (2009) Atherosclerosis and disc degeneration/low-back pain – A systematic review. Eur J Vasc Endovasc Surg 37:661–670. https://doi.org/10.1016/j.ejvs.2009.02.006
Kauppila LI, Penttilä A, Karhunen PJ, Lalu K, Hannikainen P (1994) Lumbar disc degeneration and atherosclerosis of the abdominal aorta. Spine 19:923–929. https://doi.org/10.1097/00007632-199404150-00010
Battié MC, Videman T, Gill K, Moneta GB, Nyman R, Kaprio J et al (1991) Volvo award in clinical sciences. Smoking and lumbar intervertebral disc degeneration: an MRI study of identical twins. Spine 16:1015–1021
Alpantaki K, Kampouroglou A, Koutserimpas C, Effraimidis G, Hadjipavlou A (2019) Diabetes mellitus as a risk factor for intervertebral disc degeneration: a critical review. Eur Spine J 28:2129–2144. https://doi.org/10.1007/s00586-019-06029-7
Videman T, Sarna S, Battié MC, Koskinen S, Gill K, Paananen H et al (1995) The long-term effects of physical loading and exercise lifestyles on back-related symptoms, disability, and spinal pathology among men. Spine 20:699–709. https://doi.org/10.1097/00007632-199503150-00011
Fields AJ, Ballatori A, Liebenberg EC, Lotz JC (2018) Contribution of the endplates to disc degeneration. Curr Mol Bio Rep 4:151–160. https://doi.org/10.1007/s40610-018-0105-y
Farshad-Amacker NA, Hughes A, Herzog RJ, Seifert B, Farshad M (2017) The intervertebral disc, the endplates and the vertebral bone marrow as a unit in the process of degeneration. Eur Radiol 27:2507–2520. https://doi.org/10.1007/s00330-016-4584-z
Horner HA, Urban JP (2001) Volvo Award Winner in Basic Science Studies: Effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine 26:2543–2549. https://doi.org/10.1097/00007632-200112010-00006
Ratcliffe JF (1981) The arterial anatomy of the developing human dorsal and lumbar vertebral body. A microarteriographic study. J Anat 133:625–38. https://doi.org/10.1111/(ISSN)1469-7580
Gilchrist RV, Slipman CW, Isaac Z, Lenrow DA, Chou LH (2002) Vascular supply to the lumbar spine: an intimate look at the lumbosacral nerve roots. Pain Phys 5:288–293
Kauppila LI (1994) Blood supply of the lower thoracic and lumbosacral regions: postmortem aortography in 38 young adults. Acta Radiol 35(6):541–544. https://doi.org/10.1080/02841859409173319
Volodos NL (1986) Self-fixing synthetic prosthesis for endoprosthetics of the vessels. VestnKhir (Russia) 137:123–5
Nikolay LV (2013) Historical perspective: the first steps in endovascular aortic repair: How it all began. J Endovasc Ther 20(sp1):I-3-I–23. https://doi.org/10.1583/1545-1550-20.sp1.I-3
Bryce Y, Rogoff P, Romanelli D, Reichle R (2015) Endovascular repair of abdominal aortic aneurysms: vascular anatomy, device selection, procedure, and procedure-specific complications. Radiographics 35:593–615. https://doi.org/10.1148/rg.352140045
Uflacker R, Robison J (2001) Endovascular treatment of abdominal aortic aneurysms: a review. Eur Radiol 11:739–753. https://doi.org/10.1007/s003300000747
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. https://doi.org/10.1016/j.jbi.2008.08.010
Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N (2001) Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 26:1873–1878. https://doi.org/10.1097/00007632-200109010-00011
Rajasekaran S, Venkatadass K, Babu JN, Ganesh K, Shetty AP (2008) Pharmacological enhancement of disc diffusion and differentiation of healthy, ageing and degenerated discs. Eur Spine J 17:626–643. https://doi.org/10.1007/s00586-008-0645-6
Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR (1988) Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology 166:193–199. https://doi.org/10.1148/radiology.166.1.3336678
Farshad-Amacker NA, Herzog RJ, Hughes AP, Aichmair A, Farshad M (2015) Associations between lumbosacral transitional anatomy types and degeneration at the transitional and adjacent segments. Spine J 15:1210–1216. https://doi.org/10.1016/j.spinee.2013.10.029
Hanhivaara J, Määttä JH, Niinimäki J, Nevalainen MT (2020) Lumbosacral transitional vertebrae are associated with lumbar degeneration: retrospective evaluation of 3855 consecutive abdominal CT scans. Eur Radiol 30:3409–3416. https://doi.org/10.1007/s00330-020-06691-2
White GH, Yu W, May J, Chaufour X, Stephen MS (2016) Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: classification incidence, diagnosis, and management. J Endovasc Ther 4:152–168. https://doi.org/10.1177/152660289700400207
Müller-Wille R, Borgmann T, Wohlgemuth WA, Zeman F, Pfister K, Jung EM et al (2014) Dual-energy computed tomography after endovascular aortic aneurysm repair: the role of hard plaque imaging for endoleak detection. Eur Radiol 24:2449–2457. https://doi.org/10.1007/s00330-014-3266-y
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159. https://doi.org/10.2307/2529310
Wang Y, Videman T, Battié MC (2012) ISSLS prize winner: lumbar vertebral endplate lesionsassociations with disc degeneration and back pain history. Spine 37:1490–1496. https://doi.org/10.1097/BRS.0b013e3182608ac4
Farshad-Amacker NA, Hughes AP, Aichmair A, Herzog RJ, Farshad M (2014) Determinants of evolution of endplate and disc degeneration in the lumbar spine: a multifactorial perspective. Eur Spine J 23:1863–1868. https://doi.org/10.1007/s00586-014-3382-z
Aoki J, Yamamoto I, Kitamura N, Sone T, Itoh H, Torizuka K et al (1987) End plate of the discovertebral joint: degenerative change in the elderly adult. Radiology 164:411–414. https://doi.org/10.1148/radiology.164.2.3602378
Albert HB, Briggs AM, Kent P, Byrhagen A, Hansen C, Kjaergaard K (2011) The prevalence of MRI-defined spinal pathoanatomies and their association with Modic changes in individuals seeking care for low back pain. Eur Spine J 20:1355–1362. https://doi.org/10.1007/s00586-011-1794-6
Vergauwen S, Parizel PM, van Breusegem L, Van Goethem JW, Nackaerts Y, Van den Hauwe L et al (1997) Distribution and incidence of degenerative spine changes in patients with a lumbo-sacral transitional vertebra. Eur Spine J 6:168–172. https://doi.org/10.1007/BF01301431
Zankl AR, Schumacher H, Krumsdorf U, Katus HA, Jahn L, Tiefenbacher CP (2006) Pathology, natural history and treatment of abdominal aortic aneurysms. Clin Res Cardiol 96:140–151. https://doi.org/10.1007/s00392-007-0472-5
Wang Y, Videman T, Battié MC (2012) Lumbar vertebral endplate lesions: prevalence, classification, and association with age. Spine 37:1432–1439. https://doi.org/10.1097/BRS.0b013e31824dd20a
Takeyachi Y, Yabuki S, Arai I, Midorikawa H, Hoshino S, Chiba K, Kikuchi S (2006) Changes of low back pain after vascular reconstruction for abdominal aortic aneurysm and high aortic occlusion: a retrospective study. Surg Neurol 66:172–176. https://doi.org/10.1016/j.surneu.2006.02.038
Kalichman L, Guermazi A, Li L, Hunter DJ (2009) Association between age, sex, BMI and CT-evaluated spinal degeneration features. J Back Musculoskelet Rehabil 22:189–195. https://doi.org/10.3233/BMR-2009-0232
Kalichman L, Kim DH, Li L, Guermazi A, Hunter DJ (2010) Computed tomography-evaluated features of spinal degeneration: prevalence, intercorrelation, and association with self-reported low back pain. Spine J 10:200–208. https://doi.org/10.1016/j.spinee.2009.10.018
Haughton V (2006) Imaging intervertebral disc degeneration. JBJS 88:15. https://doi.org/10.2106/JBJS.F.00010
Funding
Open access funding provided by University of Zurich.
Author information
Authors and Affiliations
Contributions
All authors edited and reviewed the text, and agreed to the last, here submitted version. NF and MF performed the text for ethical approval, NF was involved in everything, made the first draft of the manuscript, performed a readout, and edited and reviewed the text. Patient data collection was performed from NF, UM, JG, GP, TP and UM. UM also performed a readout. Statistical analyses were performed by TG under supervision of NF, MF and RS.
Corresponding author
Ethics declarations
Conflict of interest
None.
Consent to participate
This was a retrospective study design- therefore not applicable.
Consent for publication
All authors have contributed adequately to the content of the manuscript, have read it, concur with the content of it and confirm the manuscript represents honest work.
Ethical approval.
This study was approved by the ethic committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farshad-Amacker, N.A., Farshad, M., Galley, J. et al. Atypical patterns of spinal segment degeneration in patients with abdominal aortic aneurysms. Eur Spine J 32, 8–19 (2023). https://doi.org/10.1007/s00586-022-07276-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-022-07276-x