Abstract
Purpose
Quantitative comparison of diffusion parameters from various models of diffusion-weighted (DWI) and diffusion kurtosis (DKI) imaging for distinguishing spinal metastases and chordomas.
Methods
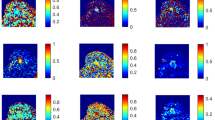
DWI and DKI examinations were performed in 31 and 13 cases of spinal metastases and chordomas, respectively. DWI derived apparent diffusion coefficient (ADC), true diffusion coefficient (D), pseudo diffusion coefficient (D*), perfusion fraction (f), water molecular distributed diffusion coefficient (DDC), and intravoxel water diffusion heterogeneity (α). DKI derived mean diffusivity (MD) and mean kurtosis (MK). Independent sample t-testing compared statistical differences among parameters. Sensitivity, specificity, and area under the receiver operating characteristic (ROC) curve were determined. Pearson correlation analysis evaluated the parameters’ correlations.
Results
ADC, D, f, DDC, α, and MD were significantly lower in spinal metastases than chordomas (all P < 0.05). MK was significantly higher in spinal metastases than chordomas (P < 0.05). D had the highest area under the ROC curve (AUC) of 0.886, greater than MD (AUC = 0.706) or DDC (AUC = 0.742) in differentiating the two tumors (both P < 0.05). Combining D with f and α statistically significantly increased the AUC for diagnosis (to 0.995) relative to D alone (P < 0.05). There was a certain correlation among DDC, ADC, and D (all P < 0.05).
Conclusions
Monoexponential, biexponential, and stretched-exponential models of DWI and DKI can potentially differentiate spinal metastases and chordomas. D combined with f and α performed best.



Similar content being viewed by others
References
Zambo I, Vesely K (2014) WHO classification of tumours of soft tissue and bone 2013: the main changes compared to the 3rd edition. Cesk Patol 50: 64–70
Wang F, Chu C, Zhao C et al (2019) Diffusion kurtosis imaging in sacroiliitis to evaluate the activity of ankylosing spondylitis. J Magn Reson Imag 49(101):108
Hui ES, Cheung MM, Qi L et al (2008) Towards better MR characterization of neural tissues using directional diffusion kurtosis analysis. Neuroimage 42:122–134
Surov A, Nagata S, Razek AA et al (2015) Comparison of ADC values in different malignancies of the skeletal musculature: a multicentric analysis. Skeletal Radiol 44:995–1000
Le Bihan D, Breton E, Lallemand D et al (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168:497–505
Le Bihan D, Breton E, Lallemand D et al (1986) MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161:401–407
Bennett KM, Schmainda KM, Bennett RT et al (2003) Characterization of continuously distributed cortical water diffusion rates with a stretched-exponential model. Magn Reson Med 50:727–734
Jensen JH, Helpern JA, Ramani A et al (2005) Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 53:1432–1440
Bai Y, Lin Y, Tian J et al (2016) Grading of gliomas by Using monoexponential, biexponential, and stretched exponential diffusion-weighted MR imaging and diffusion kurtosis MR imaging. Radiology 278:496–504
Wang F, Wang Y, Zhou Y et al (2017) Comparison between types I and II epithelial ovarian cancer using histogram analysis of monoexponential, biexponential, and stretched-exponential diffusion models. J Magn Reson Imag 46:1797–1809
Fusco R, Sansone M, Granata V et al (2019) Diffusion and perfusion MR parameters to assess preoperative short-course radiotherapy response in locally advanced rectal cancer: a comparative explorative study among Standardized Index of Shape by DCE-MRI, intravoxel incoherent motion- and diffusion kurtosis imaging-derived parameters. Abdom Radiol (NY) 44:3683–3700
Wan Q, Deng YS, Lei Q et al (2019) Differentiating between malignant and benign solid solitary pulmonary lesions: Are intravoxel incoherent motion and diffusion kurtosis imaging superior to conventional diffusion-weighted imaging? Eur Radiol 29:1607–1615
Shirota N, Saito K, Sugimoto K et al (2016) Intravoxel incoherent motion MRI as a biomarker of sorafenib treatment for advanced hepatocellular carcinoma: a pilot study. Cancer Imag 16:1
Zhu L, Pan Z, Ma Q et al (2017) Diffusion kurtosis imaging study of rectal Adenocarcinoma associated with histopathologic prognostic factors: preliminary findings. Radiology 284:66–76
Ogawa M, Kan H, Arai N et al (2019) Differentiation between malignant and benign musculoskeletal tumors using diffusion kurtosis imaging. Skeletal Radiol 48:285–292
Wu G, Xie R, Liu X et al (2019) Intravoxel incoherent motion diffusion MR and diffusion kurtosis imaging for discriminating atypical bone metastasis from benign bone lesion. Br J Radiol 92:20190119
Bourillon C, Rahmouni A, Lin C et al (2015) Intravoxel incoherent motion diffusion-weighted imaging of multiple myeloma lesions: correlation with whole-body dynamic contrast agent-enhanced MR imaging. Radiology 277:773–783
Zhao YH, Li SL, Liu ZY et al (2015) Detection of Active Sacroiliitis with Ankylosing Spondylitis through Intravoxel Incoherent Motion Diffusion-Weighted MR Imaging. Eur Radiol 25:2754–2763
Akashi M, Nakahusa Y, Yakabe T et al (2014) Assessment of aggressiveness of rectal cancer using 3-T MRI: correlation between the apparent diffusion coefficient as a potential imaging biomarker and histologic prognostic factors. Acta Radiol 55:524–531
Patterson DM, Padhani AR, Collins DJ (2008) Technology insight: water diffusion MRI–a potential new biomarker of response to cancer therapy. Nat Clin Pract Oncol 5:220–233
Hatakenaka M, Soeda H, Yabuuchi H et al (2008) Apparent diffusion coefficients of breast tumors: clinical application. Magn Reson Med Sci 7:23–29
Zhang J, Chen Y, Zhang E et al (2020) Use of monoexponential diffusion-weighted imaging and diffusion kurtosis imaging and dynamic contrast-enhanced-MRI for the differentiation of spinal tumors. Eur Spine J 29:1112–1120
Hui ES, Cheung MM, Qi L et al (2008) Advanced MR diffusion characterization of neural tissue using directional diffusion kurtosis analysis. Conf Proc IEEE Eng Med Biol Soc 2008:3941–3944
Liu C, Wang K, Chan Q et al (2016) Intravoxel incoherent motion MR imaging for breast lesions: comparison and correlation with pharmacokinetic evaluation from dynamic contrast-enhanced MR imaging. Eur Radiol 26:3888–3898
Federau C, Hagmann P, Maeder P et al (2013) Dependence of brain intravoxel incoherent motion perfusion parameters on the cardiac cycle. PLoS One 8:e72856
Winfield JM, Desouza NM, Priest AN et al (2015) Modelling DW-MRI data from primary and metastatic ovarian tumours. Eur Radiol 25:2033–2040
Wu G, Liu X, Xiong Y et al (2018) Intravoxel incoherent motion and diffusion kurtosis imaging for discriminating soft tissue sarcoma from vascular anomalies. Medicine (Baltimore) 97:e13641
Lemke A, Laun FB, Simon D et al (2010) An in vivo verification of the intravoxel incoherent motion effect in diffusion-weighted imaging of the abdomen. Magn Reson Med 64:1580–1585
Acknowledgements
This study has received funding by National Natural Science Foundation of China (No. 81971578, No.81701648) and Clinical Key Project of Peking University Third Hospital (BYSY2018007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, J., Xing, X., Wang, Q. et al. Preliminary study of monoexponential, biexponential, and stretched-exponential models of diffusion-weighted MRI and diffusion kurtosis imaging on differential diagnosis of spinal metastases and chordoma. Eur Spine J 31, 3130–3138 (2022). https://doi.org/10.1007/s00586-022-07269-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-022-07269-w