Abstract
Purpose
Early disc degeneration (DD) has been thought to be associated with loss of spine stability. However, before this can be understood in relation to back pain, it is necessary to know the relationship between DD and intervertebral motion in people without pain. This study aimed to find out if early-to-moderate DD is associated with intervertebral motion in people without back pain.
Methods
Ten pain-free adults, aged 51–71, received recumbent and weight bearing MRI scans and quantitative fluoroscopy (QF) screenings during recumbent and upright lumbar flexion. Forty individual level and 10 composite (L2-S1) radiographic and MRI DD gradings were recorded and correlated with intervertebral flexion ROM, translation, laxity and motion sharing inequality and variability for both positions.
Results
Kinematic values were similar to previous control studies. DD was evidenced up to moderate levels by both radiographic and MRI grading. Disc height loss correlated slightly, but negatively with flexion during weight bearing flexion (R = − 0.356, p = 0.0.025). Composite MRI DD and T2 signal loss evidenced similar relationships (R = − 0.305, R = − 0.267) but did not reach statistical significance (p = 0.056, p = 0.096). No significant relationships between any other kinematic variables and DD were found.
Conclusion
This study found only small, indefinite associations between early-to-moderate DD and intervertebral motion in healthy controls. Motion sharing in the absence of pain was also not related to early DD, consistent with previous control studies. Further research is needed to investigate these relationships in patients.
Similar content being viewed by others
Introduction
The role of disc degeneration (DD) in the biomechanics of chronic back pain has been unclear for many years and important questions remain outstanding. For example, it has long been theorised that early DD is associated with ‘dysfunction’, that progression is followed by an ‘unstable’ phase, and advanced degeneration brings ‘stabilisation’ [1]. However, providing evidence for this, let alone any association with pain, has proved difficult. Studies using flexion–extension radiographs and later MR imaging have failed to confirm an association between DD and abnormal movement [2, 3], probably due to an inability to provide accurate and reliable measurement of subtle intervertebral motion, let alone correlate it with DD in vivo [4].
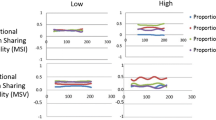
A number of studies using quantitative fluoroscopy (QF) have investigated continuous multi-segmental lumbar intervertebral motion in detail, finding quantifiable differences in motion patterns between patients with back pain and controls [5,6,7,8,9]. One considered patients with and without DD, finding more out of plane motion in the latter [10], while another found substantial correlations between DD and the degree of unequal motion sharing (MSI) during recumbent passive flexion in patients, but not in controls [8] (Fig. 1). Thus, DD is implicated not only in in vivo interactions between levels, but also in the back pain experience. This study also compared weight bearing active flexion in patients with controls and found that the variability of motion sharing (MSV) was substantially correlated with DD in patients, but not controls [8] (Fig. 1).
Derivation of MSI and MSV Example of the measurement of continuous proportional intervertebral motion during the flexion and return motion cycle of 4 intervertebral levels. Changes in angle between adjacent vertebrae are measured throughout the motion cycle a and are converted into proportional intervertebral contributions to the motion of the L2-S1 spine b. The ranges of the proportional intervertebral contributions are calculated c. Motion share inequality (MSI) was established as the mean of all the ranges throughout the flexion and return bend, and motion share variability (MSV) was the standard deviation of this range (from [8])
In a further study of CNSLBP patients and controls, individual level weight bearing MSV was found to be greater at L4-5 in patients than controls, while L5-S1 received significantly less and L2-3 more of the overall motion in patients [11]. Yet another QF study included the measurement of mid-range attainment rate, (or laxity) at each level from L2-S1 and found it not to be higher in patients with CNSLBP than normative reference limits [9].
Given these complexities, it is difficult to understand the role of DD in CNSLBP, or to evaluate the Kirkaldy-Willis and Farfan hypothesis [1]. To approach this in CNSLBP patients, we must first determine the presence or absence of associations between DD and intervertebral motion in people without pain. The aims of the present study were therefore to find out if weight bearing or recumbent MSI and MSV, flexion ROM, translation or laxity are associated with DD in pain-free controls with early-to-moderate DD.
Methods
Participants
Ten healthy participants aged between 51 and 71 years with no history of disabling back pain over the previous year were recruited from a group of pain-free volunteers who were participating in an ongoing normative QF study of recumbent and weight bearing intervertebral flexion motion. Following imaging, those who were found to have at least one intervertebral level with DD of at least Grade 2 on the Kellgren and Lawrence scale were invited to also have recumbent and weight bearing MRI scans [12]. Participant age, sex, height and weight were recorded.
Imaging
Fluoroscopic sequences were obtained using a Siemens Arcadis Avantic C-arm fluoroscope (Siemens GMBH, Germany), recording at 15fps during controlled lumbar flexion motion through 40° in the lateral decubitus position and 60° in the standing position. For recumbent screening, participants lay on a movable table whose trunk section was motorised and driven by a controller (Atlas Clinical Ltd). For standing imaging, they stood with their right side against an upright motion frame with their pelvises secured and followed a motorised arm rest which guided their flexion motion. The controllers accelerated at 6° s−2 for the first second followed by a uniform 6° s−2 thereafter. Following screening, the images were inspected by the authors (AB and FM) and all participants with at least one level with DD of at least Grade 2 also received supine and semi-recumbent sitting MRI scans on the same day. These were obtained using a Paramed MR Open 0.5 T scanner (Paramed ASG, Italy). Patients received supine and recumbent sitting T2 sagittal and axial scans from L2-S1 (Fast Spin Echo, Matrix 256 × 208, Slice thickness 5 mm, Gap 1 mm).
Image analysis
All images were inspected for incidental findings by a consultant radiologist (AM) who also performed the DD grading. The fluoroscopic sequences were exported to a computer workstation and analysed using manual image registration of the first image and thereafter bespoke frame to frame tracking codes written in MATLAB (2013—The Mathworks Ltd Cambridge). Anonymised image sequences were analysed by one operator (FM) and outputted to an Excel spreadsheet in the form of frame to frame intervertebral angular rotations throughout each motion sequence. The displacements between pairs of vertebrae were calculated using Distortion Compensated Radiographic Analysis, which is based on landmarks identified on the vertebral body ‘corners’ and provides measurement of translation independent of the position of the centre of rotation [13]. Accuracy and repeatability for intervertebral rotations, translation and laxity using this method have been determined as being high [7, 14,15,16], and these, plus MSI and MSV, were obtained from the intervertebral motion outputs [8, 17, 18].
Assessment of disc degeneration
Radiographic DD was graded 0–4 for each level by a consultant radiologist (AM) from the initial sagittal fluoroscopic image [12]. This gave a composite measure of structural change in the form of reduced disc height, osteophytes, bone sclerosis and deformation, giving a maximum composite score of 16 for the 4 intervertebral levels [8, 19].
For MRI DD, the Jarosz Atlas scale was used, employing radiologist visual assessment of both supine and weight bearing scans (Online Appendix) [20]. This unpublished 6-section tool has 5 four-point semi-quantitative scales (0–3) consisting of disc height loss, T2-weighted disc signal intensity loss, disc extension into the spinal canal, endplate marrow changes and osteophytosis. There is a further scale for alignment, scored 0 or 1, giving a maximum score per level of 16, or a maximum composite score of 64 for all 4 levels from L2-S1. In addition to the overall scores, disc height loss and signal intensity loss were included as subscales in the analysis in the absence of explicit measures of these common MRI DD variables.
Ethics
All participants gave written informed consent to their involvement in the study, which received ethical approval from the UK South West 3 Research Ethics Committee (REC Reference 10/H0106/65).
Statistical analysis
Descriptive analysis was performed for all variables, including their values during weight bearing and recumbent imaging. Following inspection for normality (Shapiro–Wilk test), the mean and maximum flexion ROM, translation, laxity MSI and MSV scores were calculated for both recumbent and weight bearing lumbar flexion and the results compared with each other (2-tailed Wilcoxon test with 5% significance) and with an existing normative dataset [21]. Weight bearing and recumbent radiographic and MRI DD scores were also compared.
Nonparametric bivariate correlations (Spearman rank correlation) were calculated for both recumbent and weight bearing flexion ROM, translation and laxity against their respective segmental DD gradings. Finally, MSI and MSV in both orientations were correlated against their composite DD scores for all participants. To interpret the relevance of the correlations obtained, an ‘R’ score of > 0.80 was considered ‘excellent, > 60–0.80 ‘substantial, 0.40–0.60 ‘moderate’ and < 0.40 ‘slight’ [22]. All data were analysed using Stats Direct statistical software (V2.07.0008, Birkenhead).
Results
Complete data were obtained for all participants, whose personal characteristics and kinematic and DD scores are shown in Table 1. The population was mainly female, average age 61, with normal BMI. The 5 kinematic variables showed similar average scores to a previous normative database study [21], and flexion ROM and MSV gave significantly larger scores on weight bearing than on recumbent QF recordings (p < = 0.01). Participants received a mean (SD) equivalent radiation dose of 0.399 mSv (0.149) from fluoroscopy, which is 0.901 mSv less than a standard radiographic investigation of the lumbar spine.
DD always scored higher when assessed from weight bearing examinations, but this only reached statistical significance for composite MRI and disc height loss (p < 0.04). However, DD was generally at the lower end of the DD ranges registering between 25 and 58% of their maximum possible scores on weight bearing assessment (lowest, radiographic DD 25%, highest, weight bearing disc height loss 58%), indicating that this population represented early-to-moderate DD. In order to optimise the range of relationships between kinematics and DD, all correlations reported here were taken using weight bearing DD assessments (Table 2).
There were no significant correlations between upright or recumbent MSI or MSV and any kind of DD, although a substantial negative correlation between recumbent MSI and radiographic DD approached significance (R = − 0.610, p = 0.06) (Table 3). Overall, this is consistent with previous studies in pain-free controls [8, 9].
There were slight negative linear correlations between disc height loss and flexion ROM (R = − 0.356, p = 0.025) and between MRI DD and flexion ROM (R = − 0.305, p = 0.056), with assessments of both motion and DD performed weight bearing. (Fig. 2a, b). However, the latter did not quite reach significance. Scatterplots of all correlations between weight bearing DD assessments and both recumbent and weight bearing kinematic variables are shown in the Supplementary Material).
Scatter plots showing correlations between ROM and radiologist weight bearing MRI assessments of a disc height loss and b overall disc degeneration (/16) [20]
Discussion
This study found a slight negative correlation between disc height reduction on MRI and flexion ROM only, as assessed during weight bearing lumbar flexion in healthy controls without back pain and with early-to-moderate DD. No correlations were found with translation or laxity or with any kinematic variable during passive recumbent motion. This finding is, if anything, the reverse of the relationship proposed by Kirkaldy-Willis and Farfan [1]. Weight bearing MRI scans returned a significantly greater loss of disc height than recumbent ones, which is consistent with a previous study that found that positional changes tend to be more frequent on weight bearing MRI scans [23]. However, a similar weak negative correlation was also found between flexion ROM and disc height loss on the recumbent MRI scans (R = − 0.350, p = 0.027).
No other significant associations were found between DD and intervertebral motion values, which were comparable to other normative studies [21]. This suggests, (but does not prove) that it may have been the motion abnormalities in the symptomatic patient studies, rather than the degenerative changes, that were the main drivers of nociceptive pain [8]. A review of post-fusion adjacent segment kinematic studies, where DD was implicated, concluded that there appears to be no overall kinematic changes at the rostral or caudal levels adjacent to a fusion [24]. Furthermore, although the levels of DD recorded in this study have been associated with the ‘dysfunctional’, or at most, ‘unstable’ phase of DD, the flexion ROMs found here were similar to other studies of pain-free participants [21].
However, cadaveric studies have found associations between neutral zone (NZ) length and radiographic DD [25], although these have also given contradictory results, where one found radiographic and MRI DD to be associated with decreased flexion ROM [26], while another found small increases in the NZ with MRI, but not with radiographic DD [27].
There are inaccuracies inherent in radiographic studies of intervertebral motion in vivo which can be largely overcome by radiostereometric analysis (RSA). However, the invasiveness of this method makes it unsuitable for use in asymptomatic controls, as well as largely inaccessible for patients with chronic, nonspecific low back pain (CNSLBP) [28]. Nevertheless, one RSA study of 18 patients with back pain being considered for lumbar fusion did not detect an ‘instability’ phase in early DD [29].
The natural successor to the present biomechanical study would be a cross-sectional clinical study along the same lines. A previous study that compared patients with treatment-resistant back pain to controls (but did not assess DD) also found the composite value of MSI to be higher in patients than controls [9]. However, laxity, translation and flexion ROM were not greater in patients. Therefore, a repeat of the present study with a larger population and a DD assessment similar to the present one (i.e. using recumbent and weight bearing QF and MRI) could tell us whether the MSI marker is linked to DD in vivo.
If similar results are found at individual levels in CNLBP patients, (i.e. little or no association between DD and IV motion), it will be evidence of an absence of direct DD involvement in the pain process, consistent with the findings of Axelsson and Karlsson [29]. However, if DD is again associated with increased MSI in recumbent examinations and is also associated with individual level weight bearing intervertebral flexion motion sharing changes as recently found in patients, this would provide evidence that the disc is not usually the nociceptive source [8, 9]. The task then would be to add the assessment of other known pain generators (e.g. muscle hypoxia, loading stresses, fatigability) to such investigations to determine the prevalence of these as nociceptive stimuli.
If we consider the relevance of the destabilisation–restabilisation theory [1] in respect of patients, it is not unexpected that there would be little relationship between intervertebral motion and DD in healthy controls, as this is consistent with the lack of correlation with radiographic DD found in other studies and its contrast with the strong correlations (R = 0.70 and 0.85) with MSI and MSV found in patients with CNSLBP [8]. What is yet to be determined is whether MSI and/or MSV are related to the MRI DD factors in patients and whether flexion ROM, translation or laxity is involved. One possible correlation with the kinematic variables presented here is disc shear stiffness, which is becoming assessable in vivo by MR elastography [30]. Other forms of imaging, such as diffusion weighted MRI, could also be explored as it may allow more complex associations with kinematics to be assessed in patients [31]. However, continuous in vivo dynamic motion assessments will be required as opposed to plain radiographs or kMRI, which only records categorical motion data from quasi-static measurements [32, 33].
Also to be considered is the siting, as well as the severity of degenerated discs when attempting to explore associations between DD, intervertebral motion and CNSLBP. Recent QF research comparing CNSLBP patients to control in terms of individual level motion sharing throughout upright flexion suggests that in patients, L5-S1 receives less and L2-3 more of the motion [11]. Given the apparent importance of motion sharing in symptoms, and in the light of the present study, it would be useful to investigate the influence of DD graded using upright MRI, on these motion distributions and their relationships to disability. This may help to explain the findings of Cheung et al. [34] who reported that pain and disability were greater in patients with ‘continuous level DD’ than ‘skipped level DD’. This has been further pursued by von Forrell et al. [35] using FE modelling and finding higher intervertebral stresses to be associated with continuous level DD compared with skipped level DD. Prospective studies of kinematics and stresses are becoming accessible using QF motion analysis along with FE models based on 3-D MRI scans which could include muscle demands [36]. Future studies of patients might therefore consider the distribution of degenerate discs along with their grades, kinematics and loading stresses in relation to such muscle demands and disability.
Limitations
The present study was limited by small participant numbers (n = 10), which reduced the power to find significant correlations between MSI, MSV and composite DD. However, the number of individual intervertebral level DD measures and kinematic markers was 40 and should have revealed true correlations if not affected by level-specific differences in DD. Indeed, a biomechanical study by Roussouly and Pinheiro-Franco proposed that there are level-specific patterns in DD [37]. However, a later study by Torrie et al. [38] found that lumbar spinal subtype, based on morphology, was not statistically significantly correlated with DD. For the composite measures of MSI, MSV and composite radiographic disc degeneration however, a larger sample will be necessary to investigate significant relationships.
This study was also confined to nonparametric linear regression analysis, by virtue of the categorical nature of the DD gradings, while the kinematic data were interval in nature. This prevented the detection of any nonlinear associations throughout the spectrum of DD severity. More advanced methods for imaging the disc quantitatively and objectively could remove these problems.
Conclusion
In an older pain-free population with early-to-moderate DD, this study found only small, indefinite associations with intervertebral mobility. Furthermore, only small and negative correlations were found between weight bearing flexion ROM and disc height loss, which is not consistent with the Kirkaldy-Willis and Farfan hypothesis [1]. No significant correlations were found between any other measure of DD and flexion ROM, translation, laxity, MSI or MSV. These relationships may be different in patients with CNSLBP.
References
Kirkaldy-Willis WH, Farfan HF (1982) Instability of the lumbar spine. Clin Orthop Relat Res 165:110–123
Paajanen H, Erkintalo M, Dahlstrom S, Kuusela T, Svedstrom E, Kormano M (1989) Disc degeneration and lumbar instability. Acta Orthop Scand 60:375–378
Murata M, Morio Y, Kuranobu K (1994) Lumbar disc degeneration and segmental instability: a comparison of magnetic resonance images and plain radiographs of patients with low back pain. Arch Orthop Trauma Surg 113:297–301
Penning L, Wilmink JT, Van Woerden HH (1984) Inability to prove instability: a critical appraisal of clinical-radiological flexion-extension studies in lumbar disc degeneration. Diagn Imaging Clin Med 53:186–192
Ahmadi A, Maroufi N, Behtash H, Zekavat H, Parnianour M (2009) Kinematic analysis of dynamic lumbar motion in patients with lumbar segmental instability using digital videofluoroscopy. Eur Spine J 18:1677–1685
Cheng B, Castellvi AE, Davis RJ, Lee DC, Lorio MP, Prosko RE, Wade C (2016) Variability in flexion extension radiographs of the lumbar spine: a comparison of uncontrolled and controlled bending. Int J Spine Surg. https://doi.org/10.14444/3020
Mellor FE, Thomas P, Thompson P, Breen AC (2014) Proportional lumbar spine inter-vertebral motion patterns: a comparison of patients with chronic non-specific low back pain and healthy controls. Eur Spine J 23:2059–2067. https://doi.org/10.1007/s00586-014-3273-3
Breen A, Breen A (2018) Uneven intervertebral motion sharing is related to disc degeneration and is greater in patients with chronic, non-specific low back pain: an in vivo, cross-sectional cohort comparison of intervertebral dynamics using quantitative fluoroscopy. Eur Spine J 27:145–153. https://doi.org/10.1007/s00586-017-5155-y
Breen A, Mellor F, Breen A (2018) Aberrant intervertebral motion in patients with treatment-resistant nonspecific low back pain: a retrospective cohort study and control comparison. Eur Spine J 27:2831–2839. https://doi.org/10.1007/s00586-018-5666-1
Cheng JS, Carr CB, Wong C, Sharma A, Mahfouz MR, Komistek RD (2013) Altered spinal motion in low back pain associated with lumbar strain and spondylosis. Evid Based Spine Care 4:6–12
Breen A, Breen AC (2020) Dynamic interactions between lumbar intervertebral motion segments during forward bending and return. J Biomech. https://doi.org/10.1016/j.jbiomech.2020.109603
Kellgren JH, Lawrence JS (1958) Osteo-arthrosis and disc degeneration in an urban population. Ann Rheum Dis 17:388–397
Frobin F, Brinckmann P, Lievseth G, Biggemann M, Reikeras O (1996) Precision measurement of segmental motion from flexion-extension radiographs of the lumbar spine. Clin Biomech 11:457–465
Breen A, Muggleton J, Mellor F (2006) An objective spinal motion imaging assessment (OSMIA): reliability, accuracy and exposure data. BMC Musculoskelet Disord 7:1–10
du Rose A, Breen A (2016) Relationships between lumbar inter-vertebral motion and lordosis in healthy adult males: a cross sectional cohort study. BMC Musculoskelet Disord 17(1):121
Breen A, Breen A (2016) Accuracy and repeatability of quantitative fluoroscopy for the measurement of sagittal plane translation and instantaneous axis of rotation in the lumbar spine. Med Eng Phys 38:607–614
Breen AC, Dupac M, Osborne N (2015) Attainment rate as a surrogate indicator of the intervertebral neutral zone length in lateral bending: An in vitro proof of concept study. Chiropr Man Ther 23:28. https://doi.org/10.1186/s12998-015-0073-8
Breen A, Claerbout E, Hemming R, Ayer R, Breen A (2019) Comparison of intra subject repeatability of quantitative fluoroscopy and static radiography in the measurement of lumbar intervertebral flexion translation. Sci Rep 9:19253. https://doi.org/10.1038/s41598-019-55905-1
Meetings of interest for spine physicians and surgeons (1986) SO—Spine 11(6):656–657
Jarosz JB, Pemberton J, Sambrook PN, Spector TD (1997) An atlas for scoring cervical and lumbar disc degeneration
Breen A, Hemming R, Mellor F, Breen A (2019) Intrasubject repeatability of in vivo intervertebral motion parameters using quantitative fluoroscopy. Eur Spine J 28:450–460. https://doi.org/10.1007/s00586-018-5849-9
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Jones M, Morris A, Pope A, Ayer R, Breen A (2016) Findings in back pain patients referred for upright MRI. Bone Joint J 98:23
Malakoutian M, Volkheimer D, Street J, Dvorak MF, Wilke HJ, Oxland TR (2015) Do in vivo kinematic studies provide insight into adjacent segment degeneration? A qualitative systematic literature review. Eur Spine J 24:1865–1881
Mimura M, Panjabi MM, Oxland TR, Crisco JJ, Yamamoto I, Vasavada A (1994) Disc Degeneration Affects the Multidirectional Flexibility of the Lumbar Spine. Spine 19:1371–1380
Kettler A, Rohlmann F, Ring C, Mack C, Wilke HJ (2011) Do early stages of lumbar intervertebral disc degeneration really cause instability? Evaluation of an in vitro database. Eur Spine J 20:578–584
Volkheimer D, Galbusera F, Liebsch C, Schlegel S, Rohlmann F, Kleiner S, Wilke HJ (2018) Is intervertebral disc degeneration related to segmental instability? An evaluation with two different grading systems based on clinical imaging. Acta Radiol 59:327–335. https://doi.org/10.1177/0284185117715284
Axelsson P, Johnson R, Stromqvist B (2000) Is there increased intervertebral mobility in isthmic adult spondylolisthesis? A matched comparative study using roentgen stereophotogrammetry. Spine 25:1701–1703
Axelsson P, Karlsson BS (2004) Intervertebral mobility in the progressive degenerative process: a radiostereometric analysis. Eur Spine J 13:567–572
Walter BA, Mageswaran P, Mo X, Boulter DJ, Mashaly H, Nguyen XV, Prevedello LM, Thoman W, Raterman BD, Kalra P (2017) MR elastography–derived stiffness: a biomarker for intervertebral disc degeneration. J Radiology 285:167–175
Beattie PF, Donley JW, Arnot CF, Miller R (2009) The change in the diffusion of water in normal and degenerative lumbar intervertebral discs following joint mobilization compared to prone lying. J Orthop Sports Phys Ther 39:4–11
Lao L, Daubs MD, Scott TP, Lord EL, Cohen JR, Tin R, Zhong G, Wang JC (2015) Effect of disc degeneration on lumbar segmental mobility analyzed by kinetic magnetic resonance imaging. Spine 40:316–322
Fujiwara A, Tamai K, An HS, Kurihashi A, Lim T, Yoshida H, Saotome K (2000) The relationship between disc degeneration, facet joint osteoarthritis and stability of the degenerative lumbar spine. J Spinal Disord 13:444–450
Cheung KMC, Samartzis D, Karppinen J, Luk KDK (2012) Are “patterns” of lumbar disc degeneration associated with low back pain?: new insights based on skipped level disc pathology. Spine 37:E430–E438. https://doi.org/10.1097/BRS.0b013e3182304dfc
Von Forell GA, Stephens TK, Samartzis D, Bowden AE (2015) Low back pain: a biomechanical rationale based on "patterns" of disc degeneration. Spine 40:1165–1172
Zanjani-Pour S, Meakin JR, Breen A, Breen A (2018) Estimation of in vivo inter-vertebral loading during motion using fluoroscopic and magnetic resonance image informed finite element models. J Biomech 70:134–139
Roussouly P, Pinheiro-Franco JL (2011) Biomechanical analysis of the spino-pelvic organization and adaptation in pathology. Eur Spine J 20:S609–S618
Torrie PAG, McKay G, Byrne R, Morris SAC, Harding J (2015) The influence of lumbar spinal subtype on lumbar intervertebral disc degeneration in young and middle-aged adults. Spine Deform 3:172–179. https://doi.org/10.1016/j.jspd.2014.08.006
Acknowledgements
This study received funding from The Radiological Research Trust and Paramed ASG. The MRI Disc Degeneration Atlas was reproduced by kind permission of Frances MK Williams PhD FRCP(E), Professor of Genomic Epidemiology, Hon Consultant Rheumatologist GSTT and Campus Dean St Thomas’ Dept Twin Research, St Thomas’ Hospital London SE1 7EH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Breen, A., Mellor, F., Morris, A. et al. An in vivo study exploring correlations between early-to-moderate disc degeneration and flexion mobility in the lumbar spine. Eur Spine J 29, 2619–2627 (2020). https://doi.org/10.1007/s00586-020-06526-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-020-06526-0