Abstract
Background
Cancer-associated fibroblasts (CAFs) are essential constituents of cancer-supportive microenvironments. The high incidence of hepatocellular carcinoma (HCC) in advanced fibrosis patients implies that fibroblasts have a promoting effect on HCC development. We aimed to explore the regulators of phenotypes and function of CAFs in the liver.
Methods
We established primary cancer-associated fibroblasts (CAFs) and non-cancerous liver fibroblasts (NFs) from 15 patients who underwent HCC resection. We compared phenotypes, capacity of cytokine/chemokine production and gene expression profiles between pairs of CAFs and NFs from the same donors. We examined resected tissue from additional 50 patients with HCC for immunohistochemical analyses.
Results
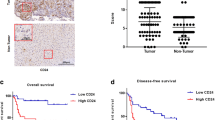
The CAFs expressed more ACTA2 and COL1A1 than the NFs, suggesting that CAFs are more activated phenotype. The CAFs produced larger amounts of IL-6, IL-8 and CCL2 than the NFs, which led to invasiveness of HuH7 in vitro. We found that Bone Morphogenetic Protein-4 (BMP4) is up-regulated in CAFs compared to NFs. The CAF phenotype and function were gained by BMP4 over-expression or recombinant BMP4 given to fibroblasts, all of which decreased with BMP4 knockdown. In tissues obtained from the patients, BMP4-positive cells are mainly observed in encapsulated fibrous lesions and HCC. Positive expression of BMP4 in HCC in resected tissues, not in fibroblasts, was associated with poorer postoperative overall survival in patients with HCC.
Conclusion
Endogenous and exogenous BMP4 activate liver fibroblasts to gain capacity of secreting cytokines and enhancing invasiveness of cancer cells in the liver. BMP4 is one of the regulatory factors of CAFs functioning in the microenvironment of HCC.





Similar content being viewed by others
Abbreviations
- BMP4:
-
Bone morphogenetic protein-4
- CAFs:
-
Cancer-associated fibroblasts
- CM:
-
Conditioned media
- DAAs:
-
Direct anti-viral agents
- HCC:
-
Hepatocellular carcinoma
- HSCs:
-
Hepatic stellate cells
- NFs:
-
Non-cancerous liver fibroblasts
- OS:
-
Overall survival
References
Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet (London, England). 2012;379:1245–55.
Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet (London, England). 2015;385:1124–35.
Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology (Baltimore, MD). 2015;62:1723–30.
El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology (Baltimore, MD). 2014;60:1767–75.
El-Serag HB, Kanwal F, Richardson P, et al. Risk of hepatocellular carcinoma after sustained virologic response in veterans with HCV-infection. Hepatology (Baltimore, Md) 2016;64:130–137.
Iwaisako K, Jiang C, Zhang M, et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci USA. 2014;111:E3297–305.
Holt AP, Salmon M, Buckley CD, et al. Immune interactions in hepatic fibrosis. Clin Liver Dis. 2008;12(861–82):x.
Franco OE, Shaw AK, Strand DW, et al. Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol. 2010;21:33–9.
Sharon Y, Raz Y, Cohen N, et al. Tumor-derived osteopontin reprograms normal mammary fibroblasts to promote inflammation and tumor growth in breast cancer. Can Res. 2015;75:963–73.
Tjomsland V, Spangeus A, Valila J, et al. Interleukin 1alpha sustains the expression of inflammatory factors in human pancreatic cancer microenvironment by targeting cancer-associated fibroblasts. Neoplasia (New York, NY). 2011;13:664–75.
Rupp C, Scherzer M, Rudisch A, et al. IGFBP7, a novel tumor stroma marker, with growth-promoting effects in colon cancer through a paracrine tumor-stroma interaction. Oncogene. 2015;34:815–25.
Lau EY, Lo J, Cheng BY, et al. Cancer-Associated fibroblasts regulate tumor-initiating cell plasticity in hepatocellular carcinoma through c-Met/FRA1/HEY1 signaling. Cell Rep. 2016;15:1175–89.
Kuchnio A, Moens S, Bruning U, et al. The cancer cell oxygen sensor PHD2 promotes metastasis via activation of cancer-associated fibroblasts. Cell Rep. 2015;12:992–1005.
Kouwaki T, Okamoto T, Ito A, et al. Hepatocyte factor JMJD5 regulates hepatitis B virus replication through interaction with HBx. J Virol. 2016;90:3530–42.
Nwani NG, Deguiz ML, Jimenez B, et al. Melanoma cells block PEDF production in fibroblasts to induce the tumor-promoting phenotype of cancer-associated fibroblasts. Can Res. 2016;76:2265–76.
Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48.
Ohlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014;211:1503–23.
Madar S, Goldstein I, Rotter V. ‘Cancer associated fibroblasts’—more than meets the eye. Trends Mol Med. 2013;19:447–53.
Salazar VS, Gamer LW, Rosen V. BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol. 2016;12:203–21.
Lee KW, Yeo SY, Sung CO, et al. Twist1 is a key regulator of cancer-associated fibroblasts. Can Res. 2015;75:73–85.
Nemer G, Nemer M. Transcriptional activation of BMP-4 and regulation of mammalian organogenesis by GATA-4 and -6. Dev Biol. 2003;254:131–48.
Kim JS, Kurie JM, Ahn YH. BMP4 depletion by miR-200 inhibits tumorigenesis and metastasis of lung adenocarcinoma cells. Mol Cancer. 2015;14:173.
Li Z, Fei T, Zhang J, et al. BMP4 Signaling Acts via dual-specificity phosphatase 9 to control ERK activity in mouse embryonic stem cells. Cell Stem Cell. 2012;10:171–82.
Ma J, Zeng S, Zhang Y, et al. BMP4 promotes oxaliplatin resistance by an induction of epithelial-mesenchymal transition via MEK1/ERK/ELK1 signaling in hepatocellular carcinoma. Cancer Lett. 2017;411:117–29.
Ma J, Zeng S, Zhang Y, et al. BMP4 enhances hepatocellular carcinoma proliferation by promoting cell cycle progression via ID2/CDKN1B signaling. Mol Carcinog. 2017;56:2279–89.
Zeng S, Zhang Y, Ma J, et al. BMP4 promotes metastasis of hepatocellular carcinoma by an induction of epithelial-mesenchymal transition via upregulating ID2. Cancer Lett. 2017;390:67–76.
Maegdefrau U, Amann T, Winklmeier A, et al. Bone morphogenetic protein 4 is induced in hepatocellular carcinoma by hypoxia and promotes tumour progression. J Pathol. 2009;218:520–9.
Daher R, Kannengiesser C, Houamel D, et al. Heterozygous mutations in BMP6 pro-peptide lead to inappropriate hepcidin synthesis and moderate iron overload in humans. Gastroenterology. 2016;150(672–83):e4.
Latour C, Besson-Fournier C, Meynard D, et al. Differing impact of the deletion of hemochromatosis-associated molecules HFE and transferrin receptor-2 on the iron phenotype of mice lacking bone morphogenetic protein 6 or hemojuvelin. Hepatology. 2016;63:126–37.
Sadlonova A, Novak Z, Johnson MR, et al. Breast fibroblasts modulate epithelial cell proliferation in three-dimensional in vitro co-culture. Breast Cancer Res. 2005;7:R46–59.
Berdiel-Acer M, Bohem ME, Lopez-Doriga A, et al. Hepatic carcinoma-associated fibroblasts promote an adaptative response in colorectal cancer cells that inhibit proliferation and apoptosis: nonresistant cells die by nonapoptotic cell death. Neoplasia (New York, NY). 2011;13:931–46.
Burton DG, Krizhanovsky V. Physiological and pathological consequences of cellular senescence. Cell Mol Life Sci. 2014;71:4373–86.
Trautwein C, Friedman SL, Schuppan D, et al. Hepatic fibrosis: concept to treatment. J Hepatol. 2015;62:S15–24.
Mederacke I, Hsu CC, Troeger JS, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823.
Acknowledgements
We thank Ms. Chizu Kanokoda for her technical assistance.
Funding
This study was supported by Grants-in-aid for Research from the National Center for Global Health and Medicine (26-shi-109 and 26A201) and by the Research Program on Hepatitis from Japan Agency for Medical Research and Development (17fk0210305h0003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mano, Y., Yoshio, S., Shoji, H. et al. Bone morphogenetic protein 4 provides cancer-supportive phenotypes to liver fibroblasts in patients with hepatocellular carcinoma. J Gastroenterol 54, 1007–1018 (2019). https://doi.org/10.1007/s00535-019-01579-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-019-01579-5