Abstract
Purpose
Although growing evidence underlines the benefits of physical activity as supportive intervention for cancer patients, sparse data are available for exercise in patients with advanced disease stages, in particular for gastrointestinal cancer (GIC) patients who experience specific disease-associated limitations. Thus, the aim of this study is to evaluate the effects of home-based moderate intensity exercise on functional capacity, activities of daily living (ADL) and body composition in patients with advanced GIC during first-line chemotherapy.
Methods
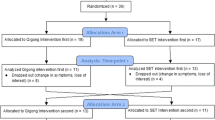
Participants (GIC, UICC III-IV; n = 44) were randomly assigned to home-based physical activity programme of 150 min moderate walking per week or a control group (CG). Functional status (SPPB: gait speed, balance, lower extremity muscle strength), postural sway, chemotherapy-induced peripheral neuropathy, nutritional state (Mini Nutritional Assessment, MNA) and lean body mass were assessed according to established recommendations. All tests were performed before chemotherapy (T0), after two chemotherapy cycles (T1) and after 12 weeks (T2).
Results
SPPB changes from T1 to T2 differed between groups with a comparably greater decrease in the CG (p < .05), but no changes or group differences over the whole study period (T0 to T2) were found. Exercise improved postural sway (T0 to T1; T0 toT2) and lean body mass (T1 to T2; T0 to T2) compared to the control group (p < .05). Gait speed, peripheral neuropathy and strength did not differ between groups (p > .05).
Conclusions
Our results indicate that a home-based physical activity improves postural sway and body composition and might stabilize functional capacity in patients with advanced GIC during chemotherapy. Although the other outcomes did not differ between groups, aforementioned effects might contribute to a maintenance of independency in ADL and a better treatment tolerance and thus enhance patients’ quality of life.




Similar content being viewed by others
References
Blanchard CM, Courneya KS, Stein K, American Cancer Society's SCS, II (2008) Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol 26(13):2198–2204. https://doi.org/10.1200/JCO.2007.14.6217
Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR (2009) Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol 10(6):598–605. https://doi.org/10.1016/S1470-2045(09)70031-2
Donohoe CL, Ryan AM, Reynolds JV (2011) Cancer cachexia: mechanisms and clinical implications. Gastroenterol Res Pract 2011:601434–601413. https://doi.org/10.1155/2011/601434
Tan BH, Fearon KC (2008) Cachexia: prevalence and impact in medicine. Curr Opin Clin Nutr Metab Care 11(4):400–407. https://doi.org/10.1097/MCO.0b013e328300ecc1
Roberts BM, Frye GS, Ahn B, Ferreira LF, Judge AR (2013) Cancer cachexia decreases specific force and accelerates fatigue in limb muscle. Biochem Biophys Res Commun 435(3):488–492. https://doi.org/10.1016/j.bbrc.2013.05.018
Fearon KC, Glass DJ, Guttridge DC (2012) Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 16(2):153–166. https://doi.org/10.1016/j.cmet.2012.06.011
Stuecher K, Bolling C, Vogt L, Dignass A, Banzer W (2016) P-208pre-therapy physical function and body status of patients with advanced gastrointestinal cancer compared to breast cancer patients and healthy women. Ann Oncol 27(suppl 2):ii60–iiii60. https://doi.org/10.1093/annonc/mdw199.200
Klepin HD, Geiger AM, Tooze JA, Newman AB, Colbert LH, Bauer DC, Satterfield S, Pavon J, Kritchevsky SB (2010) Physical performance and subsequent disability and survival in older adults with malignancy: results from the health, aging and body composition study. J Am Geriatr Soc 58(1):76–82. https://doi.org/10.1111/j.1532-5415.2009.02620.x
Brown JC, Harhay MO, Harhay MN (2015) Physical function as a prognostic biomarker among cancer survivors. Br J Cancer 112(1):194–198. https://doi.org/10.1038/bjc.2014.568
Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, von Gruenigen VE, Schwartz AL (2010) American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 42(7):1409–1426
Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH (2010) An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv 4(2):87–100. https://doi.org/10.1007/s11764-009-0110-5
Jensen W, Baumann FT, Stein A, Bloch W, Bokemeyer C, de Wit M, Oechsle K (2014) Exercise training in patients with advanced gastrointestinal cancer undergoing palliative chemotherapy: a pilot study. Support Care Cancer 22(7):1797–1806. https://doi.org/10.1007/s00520-014-2139-x
Borg G (1998) Borg's perceived exertion and pain scales. Human Kinetics
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP, American College of Sports M (2011) American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43(7):1334–1359. https://doi.org/10.1249/MSS.0b013e318213fefb
Jones LW, Eves ND, Peppercorn J (2010) Pre-exercise screening and prescription guidelines for cancer patients. Lancet Oncol 11(10):914–916. https://doi.org/10.1016/S1470-2045(10)70184-4
Castro CM, King AC (2002) Telephone-assisted counseling for physical activity. Exerc Sport Sci Rev 30(2):64–68
Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB (1994) A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49(2):M85–M94
Bohannon RW, Williams Andrews A (2011) Normal walking speed: a descriptive meta-analysis. Physiotherapy 97(3):182–189. https://doi.org/10.1016/j.physio.2010.12.004
Ruhe A, Fejer R, Walker B (2010) The test-retest reliability of centre of pressure measures in bipedal static task conditions--a systematic review of the literature. Gait Posture 32(4):436–445. https://doi.org/10.1016/j.gaitpost.2010.09.012
Pfeifer K, Ruhleder M, Brettmann K, Banzer W (2001) Effects of a coordination-focused physical activity programme on the maintenance of motor abilities of the elderly. Deut Z Sportmed 52(4):129–135
Thivolet C, el Farkh J, Petiot A, Simonet C, Tourniaire J (1990) Measuring vibration sensations with graduated tuning fork. Simple and reliable means to detect diabetic patients at risk of neuropathic foot ulceration. Diabetes Care 13(10):1077–1080
Oyer DS, Saxon D, Shah A (2007) Quantitative assessment of diabetic peripheral neuropathy with use of the clanging tuning fork test. Endocr Pract 13(1):5–10. https://doi.org/10.4158/EP.13.1.5
Pestronk A, Florence J, Levine T, Al-Lozi MT, Lopate G, Miller T, Ramneantu I, Waheed W, Stambuk M (2004) Sensory exam with a quantitative tuning fork: rapid, sensitive and predictive of SNAP amplitude. Neurology 62(3):461–464
Guigoz Y, Vellas B, Garry PJ (1996) Assessing the nutritional status of the elderly: The Mini Nutritional Assessment as part of the geriatric evaluation. Nutr Rev 54(1 Pt 2):S59–S65
Hoppe S, Rainfray M, Fonck M, Hoppenreys L, Blanc JF, Ceccaldi J, Mertens C, Blanc-Bisson C, Imbert Y, Cany L, Vogt L, Dauba J, Houede N, Bellera CA, Floquet A, Fabry MN, Ravaud A, Chakiba C, Mathoulin-Pelissier S, Soubeyran P (2013) Functional decline in older patients with cancer receiving first-line chemotherapy. J Clin Oncol 31(31):3877–3882. https://doi.org/10.1200/JCO.2012.47.7430
Bosy-Westphal A, Danielzik S, Dorhofer RP, Later W, Wiese S, Muller MJ (2006) Phase angle from bioelectrical impedance analysis: population reference values by age, sex, and body mass index. JPEN J Parenter Enteral Nutr 30(4):309–316. https://doi.org/10.1177/0148607106030004309
Segal KR, Burastero S, Chun A, Coronel P, Pierson RN,J, Wang J (1991) Estimation of extracellular and total body water by multiple-frequency bioelectrical-impedance measurement. Am J Clin Nutr 54(1):26–29
van den Dungen IA, Verhagen CA, van der Graaf WT, van den Berg JP, Vissers KC, Engels Y (2014) Feasibility and impact of a physical exercise program in patients with advanced cancer: a pilot study. J Palliat Med 17(10):1091–1098. https://doi.org/10.1089/jpm.2013.0638
Niederer D, Schmidt K, Vogt L, Egen J, Klingler J, Hubscher M, Thiel C, Bernhorster M, Banzer W (2013) Functional capacity and fear of falling in cancer patients undergoing chemotherapy. Gait Posture 39(3):865–869. https://doi.org/10.1016/j.gaitpost.2013.11.014
Quasthoff S, Hartung HP (2002) Chemotherapy-induced peripheral neuropathy. J Neurol 249(1):9–17
Streckmann F, Kneis S, Leifert JA, Baumann FT, Kleber M, Ihorst G, Herich L, Grussinger V, Gollhofer A, Bertz H (2014) Exercise program improves therapy-related side-effects and quality of life in lymphoma patients undergoing therapy. Ann Oncol 25(2):493–499. https://doi.org/10.1093/annonc/mdt568
Aaldriks AA, van der Geest LG, Giltay EJ, le Cessie S, Portielje JE, Tanis BC, Nortier JW, Maartense E (2013) Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. J Geriatr Oncol 4(3):218–226. https://doi.org/10.1016/j.jgo.2013.04.001
Ali R, Baracos VE, Sawyer MB, Bianchi L, Roberts S, Assenat E, Mollevi C, Senesse P (2016) Lean body mass as an independent determinant of dose-limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med 5(4):607–616. https://doi.org/10.1002/cam4.621
Arrieta O, Michel Ortega RM, Villanueva-Rodriguez G, Serna-Thome MG, Flores-Estrada D, Diaz-Romero C, Rodriguez CM, Martinez L, Sanchez-Lara K (2010) Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: a prospective study. BMC Cancer 10:50. https://doi.org/10.1186/1471-2407-10-50
Baracos VE (2000) Regulation of skeletal-muscle-protein turnover in cancer-associated cachexia. Nutrition 16(10):1015–1018
Llovera M, Garcia-Martinez C, Lopez-Soriano J, Agell N, Lopez-Soriano FJ, Garcia I, Argiles JM (1998) Protein turnover in skeletal muscle of tumour-bearing transgenic mice overexpressing the soluble TNF receptor-1. Cancer Lett 130(1–2):19–27
Pedersen BK (2011) Exercise-induced myokines and their role in chronic diseases. Brain Behav Immun 25(5):811–816. https://doi.org/10.1016/j.bbi.2011.02.010
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Stuecher, K., Bolling, C., Vogt, L. et al. Exercise improves functional capacity and lean body mass in patients with gastrointestinal cancer during chemotherapy: a single-blind RCT. Support Care Cancer 27, 2159–2169 (2019). https://doi.org/10.1007/s00520-018-4478-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4478-5