Abstract
Purpose
This study compared a tablet PC questionnaire with a paper method for reliability and patient preferences in the acquisition of patient-reported outcomes (PROs) for patients treated with radiotherapy. By comparing the two modes of PRO administration, we aimed to evaluate the adequacy of using tablet PC questionnaires in future clinical use.
Methods
Patients were randomized in a crossover study design using two different methods for PRO entry. A group of 89 patients answered a paper questionnaire followed by the tablet PC version, whereas 89 patients in another group completed the tablet PC questionnaire followed by the paper version. Surveys were performed four times per patient throughout the course of the radiotherapy. The Korean versions of the M.D. Anderson Symptom Inventory (MDASI-K) and the Brief Fatigue Inventory (BFI-K) were used. The primary endpoint of our current study was an assessment of patient preference for the survey method. The proportions of patients preferring each mode of questionnaire were evaluated.
Results
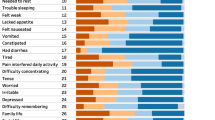
The proportion of patients who preferred the tablet PC version, paper form, or who had no preference was 52.2, 22.1, and 25.7 %, respectively. More than half of the patients preferred the tablet PC to the paper version in all four surveys. Age, gender, educational status, prior experience of using a tablet PC, and the order of paper to tablet PC administration did not impact patient preferences. Inter-class correlation coefficients (ICCs) between the modes were 0.92 for MDASI-K and 0.94 for BFI-K and ranged from 0.91 to 0.96 on both instruments during the four surveys.
Conclusions
A tablet PC-based PRO is an acceptable and reliable method compared with paper-based data collection for Korean patients receiving radiotherapy.

Similar content being viewed by others
References
Patrick DL, Guyatt GH, Acquadro C (2011) Patient-reported outcomes In: Higgins JPT, Green S (eds) Cochrane handbook for systematic reviews of interventions Version 510 [updated March 2011]. The Cochrane Collaboration www.cochrane-handbook.org. Accessed September 22 2015
Coons SJ, Gwaltney CJ, Hays RD, Lundy JJ, Sloan JA, Revicki DA, Lenderking WR, Cella D, Basch E, Force IT (2009) Recommendations on evidence needed to support measurement equivalence between electronic and paper-based patient-reported outcome (PRO) measures: ISPOR ePRO Good Research Practices Task Force report. Value Health 12:419–429
Patrick DL, Burke LB, Powers JH, Scott JA, Rock EP, Dawisha S, O’Neill R, Kennedy DL (2007) Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health 10(Suppl 2):S125–137
Fallowfield L, Jenkins V (2002) Acronymic trials: the good, the bad, and the coercive. Lancet 360:1622
Jensen RE, Rothrock NE, DeWitt EM, Spiegel B, Tucker CA, Crane HM, Forrest CB, Patrick DL, Fredericksen R, Shulman LM, Cella D, Crane PK (2015) The role of technical advances in the adoption and integration of patient-reported outcomes in clinical care. Med Care 53:153–159
Velikova G, Wright EP, Smith AB, Cull A, Gould A, Forman D, Perren T, Stead M, Brown J, Selby PJ (1999) Automated collection of quality-of-life data: a comparison of paper and computer touch-screen questionnaires. J Clin Oncol 17:998–1007
Osoba D (2011) Health-related quality of life and cancer clinical trials. Ther Adv Med Oncol 3:57–71
Sundaresan P, Sullivan L, Pendlebury S, Kirby A, Rodger A, Joseph D, Campbell I, Dhillon HM, Stockler MR (2015) Patients’ perceptions of health-related quality of life during and after adjuvant radiotherapy for T1N0M0 breast cancer. Clin Oncol (R Coll Radiol) 27:9–15
Astrup GL, Rustoen T, Miaskowski C, Paul SM, Bjordal K (2015) A longitudinal study of depressive symptoms in patients with head and neck cancer undergoing radiotherapy. Cancer Nurs 38:436–446
Guren MG, Dueland S, Skovlund E, Fossa SD, Poulsen JP, Tveit KM (2003) Quality of life during radiotherapy for rectal cancer. Eur J Cancer 39:587–594
Cella DF (1995) Methods and problems in measuring quality of life. Support Care Cancer 3:11–22
Gwaltney CJ, Shields AL, Shiffman S (2008) Equivalence of electronic and paper-and-pencil administration of patient-reported outcome measures: a meta-analytic review. Value Health 11:322–333
Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, Engstrom MC (2000) Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer 89:1634–1646
Yun YH, Mendoza TR, Kang IO, You CH, Roh JW, Lee CG, Lee WS, Lee KS, Bang SM, Park SM, Cleeland CS, Wang XS (2006) Validation study of the Korean version of the M. D Anderson Symptom Inventory J Pain Symptom Manage 31:345–352
Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL (1999) The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 85:1186–1196
Yun YH, Wang XS, Lee JS, Roh JW, Lee CG, Lee WS, Lee KS, Bang SM, Mendoza TR, Cleeland CS (2005) Validation study of the Korean version of the brief fatigue inventory. J Pain Symptom Manage 29:165–172
Valderas JM, Kotzeva A, Espallargues M, Guyatt G, Ferrans CE, Halyard MY, Revicki DA, Symonds T, Parada A, Alonso J (2008) The impact of measuring patient-reported outcomes in clinical practice: a systematic review of the literature. Qual Life Res 17:179–193
Chapman CR, Donaldson GW, Davis JJ, Bradshaw DH (2011) Improving individual measurement of postoperative pain: the pain trajectory. J Pain 12:257–262
Coons SJ, Eremenco S, Lundy JJ, O’Donohoe P, O’Gorman H, Malizia W (2015) Capturing patient-reported outcome (PRO) data electronically: the past, present, and promisE of ePRO measurement in clinical trials. Patient 8:301–309
Korea Internet & Security Agency (KISA) (2014) 2014 Korea Internet White Paper. http://isis.kisa.or.kr/eng/ebook/EngWhitePaper2014.pdf. Accessed September 22 2015
Abernethy AP, Herndon JE 2nd, Wheeler JL, Day JM, Hood L, Patwardhan M, Shaw H, Lyerly HK (2009) Feasibility and acceptability to patients of a longitudinal system for evaluating cancer-related symptoms and quality of life: pilot study of an e/tablet data-collection system in academic oncology. J Pain Symptom Manage 37:1027–1038
Zbrozek A, Hebert J, Gogates G, Thorell R, Dell C, Molsen E, Craig G, Grice K, Kern S, Hines S (2013) Validation of electronic systems to collect patient-reported outcome (PRO) data-recommendations for clinical trial teams: report of the ISPOR ePRO systems validation good research practices task force. Value Health 16:480–489
Yeomans A (2014) The future of ePRO platforms. Appl Clin Trials 23:14–19
Lawrence ST, Willig JH, Crane HM, Ye J, Aban I, Lober W, Nevin CR, Batey DS, Mugavero MJ, McCullumsmith C, Wright C, Kitahata M, Raper JL, Saag MS, Schumacher JE (2010) Routine, self-administered, touch-screen, computer-based suicidal ideation assessment linked to automated response team notification in an HIV primary care setting. Clin Infect Dis 50:1165–1173
Acknowledgments
This research was supported by a Samsung Medical Center grant (GF01130081), South Korea.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This present study was approved by the Korean Radiation Oncology Group (KROG) along with the Institutional Review Board of Ethics Committee of Samsung Medical Center, Asan Medical Center, National Cancer Center, Yeungnam University College of Medicine, and Kyung Hee University Medical Center.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kim, H., Park, H.C., Yoon, S.M. et al. Evaluation of quality of life using a tablet PC-based survey in cancer patients treated with radiotherapy: a multi-institutional prospective randomized crossover comparison of paper and tablet PC-based questionnaires (KROG 12–01). Support Care Cancer 24, 4399–4406 (2016). https://doi.org/10.1007/s00520-016-3280-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3280-5