Abstract
Objectives
The aim of this study was to investigate the incidence of febrile neutropenia (FN) with adjuvant AC (doxorubicin and cyclophosphamide) chemotherapy among Asian early-stage breast cancer (ESBC) patients, to evaluate the impact of FN on chemotherapy delivery, and to identify specific risk factors that would predispose ESBC patients to FN.
Methods
This was a single-center, observational, retrospective cohort study conducted in Singapore. All ESBC patients who have received the AC regimen as adjuvant chemotherapy between January 2007 and July 2010 were included into the study. Patients did not receive granulocyte colony-stimulating factors (G-CSF) as primary prophylaxis.
Results
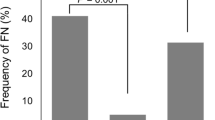
One hundred and eighty-nine patients and 729 cycles of chemotherapy were analyzed in this study, of which, majority were Chinese (84%). Median age of the patients was 54 years old (IQR 49–58). In total, 26 patients (13.8%) manifested at least one episode of FN, of which 17 patients developed FN during the first cycle of treatment. Patients who manifested FN received similar dose intensities of chemotherapy, compared to those patients who did not manifest FN (100% versus 98%, p = 0.95). After adjusting for age, race, and presence of comorbidities, low body mass index (BMI) (<23 kg/m2) was found to be associated with a higher risk of FN (OR 4.4, 95% CI = 1.65–12.01, p = 0.003).
Conclusions
Asian patients are at moderate risk for FN when they receive the AC regimen for treatment of ESBC. Further studies should evaluate the role of G-CSF to reduce the occurrence of FN in Asian patients with low BMI.
Similar content being viewed by others
References
Budman DR, Berry DA, Cirrincione CT et al (1998) Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Canc Inst 90:1205–1211
French Adjuvant Study Group (2001) Benefit of a high-dose epirubicin regimen in adjuvant chemotherapy for node-positive breast cancer patients with poor prognostic factors: 5-year follow-up results of French Adjuvant Study Group 05 randomized trial. J Clin Oncol 19:602–611
Azim HA Jr, de Azambuja E, Colozza M et al. (2011) Long-term toxic effects of adjuvant chemotherapy in breast cancer. Ann Oncol (in press)
Gianni L, Norton L, Wolmark N et al (2009) Role of anthracyclines in the treatment of early breast cancer. J Clin Oncol 27:4798–4808
Hortobagyi GN (1997) Anthracyclines in the treatment of cancer. An overview. Drugs 54(Suppl 4):1–7
Kuderer NM, Dale DC, Crawford J et al (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106:2258–2266
National Comprehensive Cancer Network. Myeloid Growth Factors. In www.nccn.org/professionals/physician_gls/pdf/myeloid_growth.pdf (accessed 2010 Sept 1), Edition.
Smith TJ, Khatcheressian J, Lyman GH et al (2006) Update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24:3187–3205
Wong AL, Chen J, Yap H et al. (2010) Correlation of ABCB1 and ABCC5 genetic variants with inter-ethnic differences in doxorubicin-induced hematologic toxicities. Proc Am Soc Clin Oncol 28: (abstr 138)
Chan A, Fu WH, Shih V et al (2011) Impact of colony-stimulating factors to reduce febrile neutropenic events in breast cancer patients receiving docetaxel plus cyclophosphamide chemotherapy. Support Care Cancer 19:497–504
Bonadonna G, Valagussa P, Moliterni A et al (1995) Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med 332:901–906
Leonard RC, Miles D, Thomas R, Nussey F (2003) Impact of neutropenia on delivering planned adjuvant chemotherapy: UK audit of primary breast cancer patients. Br J Cancer 89:2062–2068
Health Promotion Board, Singapore. Revision of Body Mass Index (BMI) Cut-offs in Singapore. In www.hpb.gov.sg/hpb/default.asp?TEMPORARY_DOCUMENT=1769&TEMPORARY_TEMPLATE=2 (accessed 2011 Mar 1), Edition
Fisher B, Anderson S, Wickerham DL et al (1997) Increased intensification and total dose of cyclophosphamide in a doxorubicin-cyclophosphamide regimen for the treatment of primary breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-22. J Clin Oncol 15:1858–1869
Vogel CL, Wojtukiewicz MZ, Carroll RR et al (2005) First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol 23:1178–1184
Timmer-Bonte JN, de Boo TM, Smit HJ et al (2005) Prevention of chemotherapy-induced febrile neutropenia by prophylactic antibiotics plus or minus granulocyte colony-stimulating factor in small-cell lung cancer: a Dutch Randomized Phase III Study. J Clin Oncol 23:7974–7984
Minisini A, Spazzapan S, Crivellari D et al (2005) Incidence of febrile neutropenia and neutropenic infections in elderly patients receiving anthracycline-based chemotherapy for breast cancer without primary prophylaxis with colony-stimulating factors. Crit Rev Oncol Hematol 53:125–131
Jenkins P, Freeman S (2009) Pretreatment haematological laboratory values predict for excessive myelosuppression in patients receiving adjuvant FEC chemotherapy for breast cancer. Ann Oncol 20:34–40
Lyman GH, Dale DC, Crawford J (2003) Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol 21:4524–4531
Barpe DR, Rosa DD, Froehlich PE (2010) Pharmacokinetic evaluation of doxorubicin plasma levels in normal and overweight patients with breast cancer and simulation of dose adjustment by different indexes of body mass. Eur J Pharm Sci 41:458–463
Joerger M, Huitema AD, Richel DJ et al (2007) Population pharmacokinetics and pharmacodynamics of doxorubicin and cyclophosphamide in breast cancer patients: a study by the EORTC-PAMM-NDDG. Clin Pharmacokinet 46:1051–1068
Acknowledgments
Authors would like to acknowledge the Department of Pharmacy, National University of Singapore for providing the Final Year Project funding for this project.
Conflict of interest
The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chan, A., Chen, C., Chiang, J. et al. Incidence of febrile neutropenia among early-stage breast cancer patients receiving anthracycline-based chemotherapy. Support Care Cancer 20, 1525–1532 (2012). https://doi.org/10.1007/s00520-011-1241-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-011-1241-6