Abstract
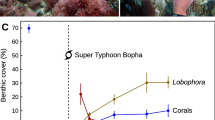
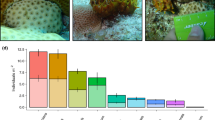
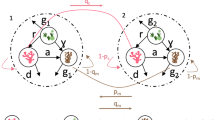
Disturbance releases space and allows the growth of opportunistic species, excluded by the old stands, with a potential to alter community dynamics. In coral reefs, abundances of fast-growing, and disturbance-tolerant sponges are expected to increase and dominate as space becomes available following acute coral mortality events. Yet, an increase in abundance of these opportunistic species has been reported in only a few studies, suggesting certain mechanisms may be acting to regulate sponge populations. To gain insights into mechanisms of population control, we simulated the dynamics of the common reef-excavating sponge Cliona tenuis in the Caribbean using an individual-based model. An orthogonal hypothesis testing approach was used, where four candidate mechanisms—algal competition, stock-recruitment limitation, whole and partial mortality—were incorporated sequentially into the model and the results were tested against independent field observations taken over a decade in Belize, Central America. We found that releasing space after coral mortality can promote C. tenuis outbreaks, but such outbreaks can be curtailed by macroalgal competition. The asymmetrical competitive superiority of macroalgae, given by their capacity to pre-empt space and outcompete with the sponge in a size-dependant fashion, supports their capacity to steal the opportunity from other opportunists. While multiple system stages can be expected in coral reefs following intense perturbation macroalgae may prevent the growth of other space-occupiers, such as bioeroding sponges, under low grazing pressure.



Similar content being viewed by others
References
Armstrong RA (1989) Fugitive coexistence in sessile species: models with continuous recruitment and determinate growth. Ecology 70:674–680
Arnold SN, Steneck R, Mumby PJ (2010) Running the gauntlet: inhibitory effects of algal turfs on the processes of coral recruitment. Mar Ecol Prog Ser 414:91. doi:10.3354/meps08724
Aronson R, Precht W, Toscano M, Koltes K (2002) The 1998 bleaching event and its aftermath on a coral reef in Belize. Mar Biol 141:435–447
Bak RP, Nieuwland G, Meesters EH (2005) Coral reef crisis in deep and shallow reefs: 30 years of constancy and change in reefs of Curacao and Bonaire. Coral Reefs 24:475–479. doi:10.1007/s00338-005-0009-1
Berger U, Piou C, Schiffers K, Grimm V (2008) Competition among plants: concepts, individual-based modelling approaches, and a proposal for a future research strategy. Perspect Plant Ecol Evolut Syst 9:121–135. doi:10.1016/j.ppees.2007.11.002
Box SJ, Mumby PJ (2007) Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar Ecol Prog Ser 342:139–149. doi:10.3354/meps342139
Burkepile DE, Hay ME (2006) Herbivore vs. nutrient control of marine primary producers: context-dependent effects. Ecology 87:3128–3139
Carballo JL (2006) Effect of natural sedimentation on the structure of tropical rocky sponge assemblages. Ecoscience 13:119–130. doi:10.2980/1195-6860(2006)13[119:eonsot]2.0.co;2
Cebrian E (2010) Grazing on coral reefs facilitates growth of the excavating sponge Cliona orientalis (Clionaidae, Hadromerida). Mar Ecol 31:533–538. doi:10.1111/j.1439-0485.2010.00401.x
Cebrian E, Uriz MJ (2006) Grazing on fleshy seaweeds by sea urchins facilitates sponge Cliona viridis growth. Mar Ecol Prog Ser 323:83–89. doi:10.3354/meps323083
Chadwick NE, Morrow KM (2011) Competition among sessile organisms on coral reefs. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Amsterdam, pp 347–371
Chamberlain SA, Bronstein JL, Rudgers JA (2014) How context dependent are species interactions? Ecol Lett 17:881–890
Chollett I, Mumby PJ (2012) Predicting the distribution of Montastraea reefs using wave exposure. Coral Reefs 31:493–503. doi:10.1007/s00338-011-0867-7
Connell JH (1983) On the prevalence and relative importance of interspecific competition: evidence from field experiments. Am Nat 122:661–696. doi:10.1086/284165
Connell JH, Hughes TE, Wallace CC, Tanner JE, Harms KE, Kerr AM (2004) A long-term study of competition and diversity of corals. Ecol Monogr 74:179–210. doi:10.1890/02-4043
Darling ES, Alvarez-Filip L, Oliver TA, McClanahan TR, Côté IM (2012) Evaluating life-history strategies of reef corals from species traits. Ecol Lett 15:1378–1386. doi:10.1111/j.1461-0248.2012.01861.x
de Goeij JM et al (2013) Surviving in a marine desert: the sponge loop retains resources within coral reefs. Science 342:108–110
Díaz MC, Rützler K (2001) Sponges: an essential component of Caribbean coral reefs. Bull Mar Sci 69:535–546
Doropoulos C, Roff G, Bozec Y-M, Zupan M, Werminghausen J, Mumby PJ (2015) Characterising the ecological trade-offs throughout the early ontogeny of coral recruitment. Ecol Monogr “Preprint”. doi: 10.1890/15-0668.1
Edwards KF, Schreiber SJ (2010) Preemption of space can lead to intransitive coexistence of competitors. Oikos 119:1201–1209. doi:10.1111/j.1600-0706.2009.18068.x
Ferrari R, Gonzalez-Rivero M, Mumby PJ (2012a) Size matters in competition between corals and macroalgae. Mar Ecol Prog Ser 467:77. doi:10.3354/meps09953
Ferrari R, Gonzalez-Rivero M, Ortiz JC, Mumby PJ (2012b) Interaction of herbivory and seasonality on the dynamics of Caribbean macroalgae. Coral Reefs 31:683–692. doi:10.1007/s00338-012-0889-9
Gardner TA, Cote IM, Gill JA, Grant A, Watkinson AR (2003) Long-term region-wide declines in Caribbean corals. Science 301:958–960. doi:10.1126/science.1086050
González-Rivero M, Yakob L, Mumby PJ (2011) The role of sponge competition on coral reef alternative steady states. Ecol Model 222:1847–1853. doi:10.1016/j.ecolmodel.2011.03.020
González-Rivero M, Ferrari R, Schönberg CHL, Mumby PJ (2012) Impacts of macroalgal competition and parrotfish predation on the growth of a common bioeroding sponge. Mar Ecol Prog Ser 444:133–142. doi:10.3354/meps09424
González-Rivero M, Ereskovsky AV, Schönberg CHL, Ferrari R, Fromont J, Mumby PJ (2013) Life-history traits of a common Caribbean coral-excavating sponge, Cliona tenuis (Porifera: Hadromerida). J Nat Hist 47:2815–2834. doi:10.1080/00222933.2013.802042
Haddon M (2001) Modelling and quantitative methods in fisheries, 2nd edn. CRC, Florida
Hastings A (1980) Disturbance, coexistence, history, and competition for space. Theor Popul Biol 18:363–373. doi:10.1016/0040-5809(80)90059-3
Hixon MA, Pacala SW, Sandin SA (2002) Population regulation: historical context and contemporary challenges of open vs. closed systems. Ecology 83:1490–1508. doi:10.1890/0012-9658
Horn HS, MacArthur RH (1972) Competition among fugitive species in a harlequin environment. Ecology 53:749–752. doi:10.2307/1934797
Hughes TP (1994) Catastrophes, phase-shifts, and large-scale degradation of a Caribbean coral reef. Science 265:1547–1551. doi:10.1126/science.265.5178.1547
Hughes TP, Connell JH (1987) Population-dynamics based on size or age—a reef-coral analysis. Am Nat 129:818–829
Hughes TP, Jackson JBC (1985) Population dynamics and life histories of foliaceous corals. Ecol Monogr 55:141–166
Hutchinson GE (1951) Copepodology for the onithologist. Ecology 32:571–577. doi:10.2307/1931745
Karlson RH, Jackson JB (1981) Competitive networks and community structure: a simulation study. Ecology 62:670–678. doi:10.2307/1937735
Kennedy E et al (2013) Avoiding coral reef functional collapse requires local and global action. Curr Biol 23:912–918. doi:10.1016/j.cub.2013.04.020
Kirchner TB, Anderson RV, Ingham RE (1980) Natural selection and the distribution of nematode sizes. Ecology 61:232–237
Knowlton N, Jackson JB (2008) Shifting baselines, local impacts, and global change on coral reefs. PLoS Biol 6:e54. doi:10.1371/journal.pbio.0060054
Lenssen JPM, van de Steeg HM, de Kroon H (2004) Does disturbance favour weak competitors? Mechanisms of changing plant abundance after flooding. J Veg Sci 15:305–314. doi:10.1658/1100-9233(2004)015
Lopez-Legentil S, Pawlik JR (2009) Genetic structure of the Caribbean giant barrel sponge Xestospongia muta using the I3-M11 partition of COI. Coral Reefs 28:157–165. doi:10.1007/s00338-008-0430-3
Lopez-Victoria M, Zea S (2005) Current trends of space occupation by encrusting excavating sponges on Colombian coral reefs. Mar Ecol 26:33–41. doi:10.1111/j.1439-0485.2005.00036.x
López-Victoria M, Zea S (2004) Storm-mediated coral colonization by an excavating Caribbean sponge. Clim Res 26:251–256. doi:10.3354/cr026251
López-Victoria M, Zea S, Weil E (2006) Competition for space between encrusting excavating Caribbean sponges and other coral reef organisms. Mar Ecol Prog Ser 312:113–121. doi:10.3354/meps312113
Mariani S, Uriz M-J, Turon X, Alcoverro T (2006) Dispersal strategies in sponge larvae: integrating the life history of larvae and the hydrologic component. Oecologia 149:174–184. doi:10.1007/s00442-006-0429-9
Muko S, Sakai K, Iwasa Y (2001) Size distribution dynamics for a marine sessile organism with space-limitation in growth and recruitment: application to a coral population. J Anim Ecol 70:579–589. doi:10.1046/j.1365-2656.2001.00513.x
Mumby PJ (1999) Bleaching and hurricane disturbances to populations of coral recruits in Belize. Mar Ecol Prog Ser 190:27–35. doi:10.3354/meps190027
Mumby PJ (2006) The impact of exploiting grazers (Scaridae) on the dynamics of Caribbean coral reefs. Ecol Appl 16:747–769. doi:10.1890/1051-0761(2006)016[0747:tioegs]2.0.co;2
Mumby PJ, Harborne AR (2010) Marine reserves enhance the recovery of corals on Caribbean reefs. PLoS One 5:e8657. doi:10.1371/journal.pone.0008657
Mumby PJ, Foster N, Fahy E (2005) Patch dynamics of coral reef macroalgae under chronic and acute disturbance. Coral Reefs 24:681–692. doi:10.1007/s00338-005-0058-5
Mumby PJ, Hastings A, Edwards HJ (2007) Thresholds and the resilience of Caribbean coral reefs. Nature 450:98–101. doi:10.1038/nature06252
Mumby PJ et al (2008) Coral reef habitats as surrogates of species, ecological functions, and ecosystem services. Conserv Biol 22:941–951. doi:10.1111/j.1523-1739.2008.00933.x
Mumby PJ et al (2011) Fishing down a Caribbean food web relaxes trophic cascades. Mar Ecol Prog Ser 445:13–24
Mumby PJ, Steneck RS, Hastings A (2013) Evidence for and against the existence of alternate attractors on coral reefs. Oikos 122:481–491
Mumby PJ, Steneck RS, Adjeroud M, Arnold SN (2015) High resilience masks underlying sensitivity to algal phase shifts of Pacific coral reefs. Oikos. doi:10.1111/oik.02673
Nelder JA, Wedderburn RWM (1972) Generalized linear models. J R Stat Soc Ser A (General) 135:370–384. doi:10.2307/2344614
Norström AV, Nyström M, Lokrantz J, Folke C (2009) Alternative states on coral reefs: beyond coral-macroalgal phase shifts. Mar Ecol Prog Ser 376:295–306. doi:10.3354/meps07815
Nugues MM, Bak RPM (2006) Differential competitive abilities between Caribbean coral species and a brown alga: a year of experiments and a long-term perspective. Mar Ecol Prog Ser 315:75–86. doi:10.3354/meps315075
Paine RT (1988) Habitat suitability and local population persistence of the sea palm Postelsia palmaeformis. Ecology 69:1787–1794. doi:10.2307/1941157
Paul V, Kuffner I, Walters L, Ritson-Williams R, Beach K, Becerro M (2011) Chemically mediated interactions between macroalgae Dictyota spp. and multiple life-history stages of the coral Porites astreoides. Mar Ecol Prog Ser 426:161–170. doi:10.3354/meps09032
Pawlik JR, Loh T-L, McMurray SE, Finelli CM (2013) Sponge communities on Caribbean coral reefs are structured by factors that are top-down, not bottom-up. PLoS One 8:e62573
Perry CT et al (2014) Changing dynamics of Caribbean reef carbonate budgets: emergence of reef bioeroders as critical controls on present and future reef growth potential. Proc R Soc B Biol Sci 281:20142018
Peterson I, Wroblewski JS (1984) Mortality rate of fishes in the pelagic ecosystem. Can J Fish Aquat Sci 41:1117–1120. doi:10.1139/f84-131
Petraitis PS, Hoffman C (2010) Multiple stable states and relationship between thresholds in processes and states. Mar Ecol Prog Ser 413:189–200. doi:10.3354/meps08691
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-PLUS. Springer, New York
Platt WJ, Weis IM (1985) An experimental study of competition among fugitive prairie plants. Ecology 66:708–720. doi:10.2307/1940532
Rasher DB, Hay ME (2010) Chemically rich seaweeds poison corals when not controlled by herbivores. PNAS 107:9683–9688. doi:10.1073/pnas.0912095107
Renken H, Mumby PJ, Matsikis I, Edwards HJ (2010) Effects of physical environmental conditions on the patch dynamics of Dictyota pulchella and Lobophora variegata on Caribbean coral reefs. Mar Ecol Prog Ser 403:63–74. doi:10.3354/meps08441
Roff G, Jx Zhao, Mumby PJ (2015) Decadal-scale rates of reef erosion following El Niño-related mass coral mortality. Glob Change Biol 21:4415–4424. doi:10.1111/gcb.13006
Rogers CS (1990) Responses of coral reefs and reef organisms to sedimentation. Mar Ecol Progr Ser 62:185–202. doi:10.3354/meps062185
Rogers CS (1993) Hurricanes and coral reefs: the intermediate disturbance hypothesis revisited. Coral Reefs 12:127–137. doi:10.1007/bf00334471
Roughgarden J, Iwasa Y, Baxter C (1985) Demographic theory for an open marine population with space-limited recruitment. Ecology 66:54–67. doi:10.2307/1941306
Rützler K (1974) The burrowing sponges of Bermuda. Smithson Contrib Zool 165:1–32. doi:10.5479/si.00810282.165
Rützler K (2002) Impact of crustose clionid sponges on Caribbean reef corals. Acta Geol Hisp 37:61–72
Sammarco PW, Risk MJ, Rose C (1987) Effects of grazing and damselfish territoriality on internal bioerosion of dead corals: indirect effects. J Exp Mar Biol Ecol 112:185–199
Sandin SA, McNamara DE (2012) Spatial dynamics of benthic competition on coral reefs. Oecologia 168:1079–1090. doi:10.1007/s00442-011-2156-0
Schoener TW (1983) Field experiments on interspecific competition. Am Nat 122:240–285. doi:10.1086/284133
Schönberg CHL (2015) Happy relationships between marine sponges and sediments—a review and some observations from Australia. J Mar Biol Assoc UK 1–22. doi: 10.1017/S0025315415001411
Schönberg CHL, Ortiz JC (2009) Is sponge bioerosion increasing? In: Proceedings of the 11th International Coral Reef Symposium, vol 1, Ft. Lauderdale, Florida, pp 527–530
Schönberg CHL, Suwa R, Hidaka M, Loh WKW (2008) Sponge and coral zooxanthellae in heat and light: preliminary results of photochemical efficiency monitored with pulse amplitude modulated fluorometry. Mar Ecol 29:1–12. doi:10.1111/j.1439-0485.2007.00216.x
Scoffin TP et al (1980) Calcium-carbonate budget of a fringing-reef on the west-coast of Barbados. 2. Erosion, sediments and internal structure. Bull Mar Sci 30:475–508
Steneck RS, Arnold SN, Mumby PJ (2014) Experiment mimics fishing on parrotfish: insights on coral reef recovery and alternative attractors. Mar Ecol Prog Ser 506:115–127
Suding KN, Goldberg D (2001) Do disturbances alter competitive hierarchies? Mechanisms of change following gap creation. Ecology 82:2133–2149. doi:10.1890/0012-9658(2001)082[2133:ddachm]2.0.co;2
van Steveninck EDD, Vanmulekom LL, Breeman AM (1988) Growth-inhibition of Lobophora variegata (Lamouroux) Womersley by scleractinian corals. J Exp Mar Biol Ecol 115:169–178. doi:10.1016/0022-0981(88)90101-3
Vicente VP (1990) Response of sponges with autotrophic endosymbionts during the coral-bleaching episode in Puerto Rico. Coral Reefs 8:199–202. doi:10.1007/bf00265011
Ward-Paige CA, Risk MJ, Sherwood OA, Jaap WC (2005) Clionid sponge surveys on the Florida Reef Tract suggest land-based nutrient inputs. Mar Pollut Bull 51:570–579. doi:10.1016/j.marpolbul.2005.04.006
Weiner J (1990) Asymmetric competition in plant populations. Trends Ecol Evol 5:360–364. doi:10.1016/0169-5347(90)90095-U
Wild C et al (2011) Climate change impedes scleractinian corals as primary reef ecosystem engineers. Mar Freshwater Res 62:205–215. doi:10.1071/mf10254
Williams EH, Bartels PJ, Bunkley-Williams L (1999) Predicted disappearance of coral-reef ramparts: a direct result of major ecological disturbances. Glob Change Biol 5:839–845
Williams ID, Polunin NVC, Hendrick VJ (2001) Limits to grazing by herbivorous fishes and the impact of low coral cover on macroalgal abundance on a coral reef in Belize. Mar Ecol Prog Ser 222:187–196
Wit E, Evd Heuvel, Romeijn J-W (2012) ‘All models are wrong…’: an introduction to model uncertainty. Stat Neerl 66:217–236. doi:10.1111/j.1467-9574.2012.00530.x
Zea S, Weil E (2003) Taxonomy of the Caribbean excavating sponge species complex Cliona caribbaea—C. aprica—C. langae (Porifera, Hadromerida, Clionaidae). Caribb J Sci 39:348–370
Acknowledgments
The authors would like to thank the contribution of five anonymous reviewers and the handling editor, S. Sandin, to the final version of the manuscript. This study was funded by: (1) the Fondo Nacional de Ciencia, Tecnología e Innovación to MGR, (2) the Wildlife Conservation Society and Khaled bin Sultan Living Oceans Foundation to R. F., and (3) the Natural Environment Research Council and ARC Laureate Fellowship to P. J. M. We are grateful to the WCS Belize staff and volunteers for their generous field assistance, and to S. O’Farrell, A. Harborne, E. Johnston and C. Doropoulos for editorial comments.
Author contribution statement
M. G. R. and P. J. M. conceived and designed the field surveys and the ecosystem model. M. G. R., R. F. and P. J. M. performed the field surveys. M. G. R., Y. M. B., I. C. and P. J. M. designed and performed the data analysis. M. G. R., P. J. M., I. C. and Y. M. B. wrote the manuscript; other authors provided editorial advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Stuart Sandin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
González-Rivero, M., Bozec, YM., Chollett, I. et al. Asymmetric competition prevents the outbreak of an opportunistic species after coral reef degradation. Oecologia 181, 161–173 (2016). https://doi.org/10.1007/s00442-015-3541-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3541-x