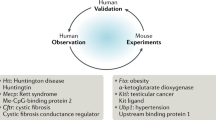
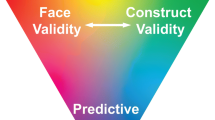
Abstract
Considering high drug attrition rates in clinical studies and the overall complexity and challenging environment of drug development, it is increasingly important to understand the therapeutic molecule and target and how they intersect with disease biology as fully as possible. This requires one to use numerous tools and investigative approaches in combination. Genetically engineered mouse models are a critical component to the drug development toolbox as they can provide key insights across multiple steps of the drug development process. While knock-out and knock-in mice can inform questions of basic biology, genetically engineered mice can also be applied to model diseases for efficacy studies, to discriminate on-target and off-target effects of novel therapeutics, and to inform an array of biologic and pharmacologic questions, including pharmacodynamics, pharmacokinetics, and biomarker discovery. However, use of these models requires not only an understanding of their strengths and limitations but also a careful consideration of the context in which they are being used and the hypotheses being addressed by them. Additionally, they should not be used in isolation, but instead in combination with other biochemical, in vitro, and clinical data to create a broad understanding of the drug, target, and disease biology.
Similar content being viewed by others
References
Alden CL, Lynn A, Bourdeau A, Morton D, Sistare FD, Kadambi VJ, Silverman L (2011) A critical review of the effectiveness of rodent pharmaceutical carcinogenesis testing in predicting for human risk. Vet Pathol 48:772–784
Alexander GM, Erwin KL, Byers N, Deitch JS, Augelli BJ, Blankenhorn EP, Heiman-Patterson TD (2004) Effect of transgene copy number on survival in the G93A SOD1 transgenic mouse model of ALS. Brain Res Mol Brain Res 130:7–15
Barrow P, Villabruna L, Hoberman A, Bohrmann B, Richter WF, Schubert C (2017) Reproductive and developmental toxicology studies with gantenerumab in PS2APP transgenic mice. Reprod Toxicol 73:362–371
Begley CG, Ellis LM (2012) Raise standards for preclinical cancer research. Nature 483:531–533
Bissig KD, Han W, Barzi M, Kovalchuk N, Ding L, Fan X, Pankowicz FP, Zhang QY, Ding X (2018) P450-humanized and human liver chimeric mouse models for studying xenobiotic metabolism and toxicity. Drug Metab Dispos 46:1734–1744
Bohin N, Carlson EA, Samuelson LC (2018) Genome toxicity and impaired stem cell function after conditional activation of CreERT2 in the intestine. Stem Cell Reports 11:1337–1346
Bohrmann B, Baumann K, Benz J, Gerber F, Huber W, Knoflach F, Messer J, Oroszlan K, Rauchenberger R, Richter WF, Rothe C, Urban M, Bardroff M, Winter M, Nordstedt C, Loetscher H (2012) Gantenerumab: a novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-β. J Alzheimers Dis 28:49–69
Boverhof DR, Chamberlain MP, Elcombe CR, Gonzalez FJ, Heflich RH, Hernández LG, Jacobs AC, Jacobson-Kram D, Luijten M, Maggi A, Manjanatha MG, Jv B, Gollapudi BB (2011) Transgenic animal models in toxicology: historical perspectives and future outlook. Toxicol Sci 121:207–233
Brayton C, Justice M, Montgomery CA (2001) Evaluating mutant mice: anatomic pathology. Vet Pathol 38:1–19
Brocard J, Warot X, Wendling O, Messaddeq N, Vonesch JL, Chambon P, Metzger D (1997) Spatio-temporally controlled site-specific somatic mutagenesis in the mouse. Proc Natl Acad Sci U S A 94:14559–14563
Bussiere JL (2008) Species selection considerations for preclinical toxicology studies for biotherapeutics. Expert Opin Drug Metab Toxicol 4(7):871–877
Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, Stone CD, Brunt EM, Xavier RJ, Sleckman BP, Li E, Mizushima N, Stappenbeck TS, Virgin HW IV (2008) A key role for autophagy and the autophagy gene Atg16L1 in mouse and human intestinal Paneth cells. Nature 456:259–264
Cadwell K, Patel KK, Maloney NS, Liu TC, Ng ACY, Storer CE, Head RD, Xavier R, Stappenbeck TS, Virgin HW (2010) Virus-plus-susceptibility gene interaction determines Crohn’s gene Atg16L1 phenotypes in intestine. Cell 141:1135–1145
Capecchi MR (2005) Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat Rev Genet 6:507–512
Catenacci DVT, Junttila MR, Karrison T, Bahary N, Noriba MN, Nattam SR, Marsh R, Wallace J, Kozloff M, Rajdev L, Cohen D, Wade J, Sleckman B, Lenz HJ, Stiff P, Kumar P, Xu P, Henderson L, Takebe N, Salgia R, Wang X, Stadler WM, de Sauvage FJ, Kindler HL (2015) Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol 33:4284–4292
Cavagnaro JA (2008) Chapter 33: Considerations in design of preclinical safety evaluation to support human cell-based therapies. In: Cavagnaro JA (ed) Preclinical safety evaluation of biopharmaceuticals: a science-based approach to facilitating clinical trials. John Wiley & Sons Inc., Hoboken, pp 749–782
Chung WJ, Daemen A, Cheng JH, Long JE, Cooper JE, Wang B, Tran C, Singh M, Gnad F, Modrusan Z, Foreman O, Junttila MR (2017) Kras mutant genetically engineered mouse models of human cancers are genomically heterogeneous. Proc Natl Acad Sci U S A 114:E10947–E10955
Cook D, Brown D, Alexander R, March R, Morgan P, Satterhwaite G, Pangalos MN (2014) Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework. Nat Rev Drug Discov 13:419–431
Dal Canto MC, Gurney ME (1995) Neuropathological changes in two lines of mice carrying a transgene for mutant human Cu,Zn SOD, and in mice overexpressing wild type human SOD: a model of familial amyotrophic lateral sclerosis (FALS). Brain Res 676:25–40
Deitch JS, Alexander GM, Bensinger A, Yang S, Jiang JT, Heiman-Patterson TD (2014) Phenotype of transgenic mice carrying a very low copy number of the mutant human G93A superoxide dismutase-1 gene associated with amyotrophic lateral sclerosis. PLoS One 9:e99879
Dey A, Seshasayee D, Noubade R, French DM, Liu J, Chaurushiya MS, Kirkpatrick DS, Pham VC, Lill JR, Bakalarski CE, Wu J, Phu L, Katavolos P, LaFave LM, Abdel-Wahab O, Modrusan Z, Seshagiri S, Dong K, Lin Z, Balazs M, Suriben R, Newton K, Hymowitz S, Garcia-Manero G, Martin F, Levine RL, Dixit VM (2012) Loss of the tumor suppressor BAP1 causes myeloid transformation. Science 337:1541–1546
Diaz, Maher (2016) Chapter 19: the use of genetically modified animals in discovery toxicology. In: Will Y, McDuffie JE, Olaharski AJ, Jeffy BD (eds) Drug discovery toxicology: from target assessment to translational biomarkers. John Wiley & Sons, Inc., Hoboken, pp 298–313
DiMasi JA, Grabowski HG, Hansen RW (2016) Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ 47:20–33
Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA Jr, Butel JS, Bradley A (1992) Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356:215–221
Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buée L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S (1996) Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature 383:710–713
Eastmond DA, Vulimiri SV, French JE, Sonawane B (2013) The use of genetically modified mice in cancer risk assessment: challenges and limitations. Crit Rev Toxicol 43:611–631
El Marjou F, Janssen KP, Chang BHJ, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S (2004) Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39:186–193
Erickson RI, Schutt LK, Tarrant JM, McDowell M, Liu L, Johnson AR, Lewin-Koh SC, Hedehus M, Ross J, Carano RA, Staflin K, Zhong F, Crawford JJ, Zhong S, Reif K, Katewa A, Wong H, Young WB, Dambach DM, Misner DL (2016) Bruton's tyrosine kinase small molecule inhibitors induce a distinct pancreatic toxicity in rats. J Pharmacol Exp Ther 360:226–238
Feil R, Brockard J, Mascrez B, LeMeur M, Metzger D, Chambon P (1996) Ligand-activated site specific recombination in mice. Proc Natl Acad Sci U S A 93:10887–10890
Feil R, Wagner J, Metzger D, Chambon P (1997) Regulation of cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun 237:752–757
Fitzgerald K, Bergeron M, Willits C, Bowers S, Aubele DL, Goldbach E, Tonn G, Ness D, Olaharski A (2013) Pharmacological inhibition of polo like kinase 2 (PLK2) does not cause chromosomal damage or result in the formation of micronuclei. Toxicol Appl Pharmacol 269:1–7
Franklin CL, Ericcson AC (2017) Microbiota and reproducibility of rodent models. Lab Anim (NY) 46:114–122
Fuji RN, Flagella M, Baca M, Baptista MA, Brodbeck J, Chan BK, Fiske BK, Honigberg L, Jubb AM, Katavolos P, Lee DW, Lewin-Koh SC, Lin T, Liu X, Liu S, Lyssikatos JP, O'Mahony J, Reichelt M, Roose-Girma M, Sheng Z, Sherer T, Smith A, Solon M, Sweeney ZK, Tarrant J, Urkowitz A, Warming S, Yaylaoglu M, Zhang S, Zhu H, Estrada AA, Watts RJ (2015) Effect of selective LRRK2 kinase inhibition on nonhuman primate lung. Sci Transl Med 7:273ra15
Galbreath EJ, Pinkert CA, Bolon B, Morton D (2013) Volume I, chapter 12: genetically engineered animals in product discovery and development. In: Haschek WM, Rousseaux CG, Wallig MA (eds) Haschek and Rousseaux’s handbook of toxicologic pathology, 3rd edn. Elsevier, Amsterdam, pp 405–460
Georgiades P, Ogilvy S, Duval H, Licence DR, Charnock-Jones SD, Smith SK, Cristin GP (2002) vavCRE transgenic mice: a tool for mutagenesis in hematopoietic and endothelial lineages. Genesis 34:251–256
Goker-Alpan O, Hruska KS, Orvisky E, Kishnani PS, Stubblefield BK, Schiffmann R, Sidransky E (2005) Divergent phenotypes in Gaucher disease implicate the role of modifiers. J Med Genet 42:e37
Goldring CE, Duffy PA, Benvenisty N, Andrews PW, Ben-David U, Eakins R, French N, Hanley NA, Kelly L, Kitteringham NR, Kurth J, Ladenheim D, Laverty H, McBlane J, Narayanan G, Patel S, Reinhardt J, Rossi A, Sharpe M, Park BK (2011) Assessing the safety of stem cell therapeutics. Cell Stem Cell 8:618–628
Gould SE, Junttila MR, de Sauvage FJ (2015) Translational value of mouse models in oncology drug development. Nat Med 21:431–439
Greenblatt MS, Bennett WP, Hollstein M, Harris CC (1994) Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res 54:4855–4878
Greenspan A, Marty-Ethgen P, Fakharzadeh S (2018) Risk of malignancies associated with ustekinumab. Br J Dermatol 178:299–300
Grubb BR, Gabriel SE (1997) Intestinal physiology and pathology in gene-targeted mouse models of cystic fibrosis. Am J Phys 273:G258–G266
Guan C, Ye C, Yang X, Gao J (2010) A review of current large-scale mouse knockout efforts. Genesis 48:73–85
Guerra C, Barbacid M (2013) Genetically engineered mouse models of pancreatic adenocarcinoma. Mol Oncol 7:232–247
Guilbault C, Saeed Z, Downey GP, Radzioch D (2007) Cystic fibrosis mouse models. Am J Respir Cell Mol Biol 36:1–7
Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, Chen W, Zhai P, Sufit RL, Siddique T (1994) Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264:1772–1775
Hansen LA, Tennant RW (1994) Follicular origin of epidermal papillomas in v-Ha-ras transgenic TG.AC mouse skin. Proc Natl Acad Sci U S A 91:7822–7826
Heffner CS, Pratt CH, Babiuk RP, Sharma Y, Rockwood SF, Donahue LR, Eppig JT, Murray SA (2012) Supporting conditional mouse mutagenesis with a comprehensive Cre characterization resource. Nat Commun 3:1218
Heger K, Wickliffe KE, Ndoja A, Zhang J, Murthy A, Dugger DL, Maltzmann A, de Sousa e Melo F, Hung J, Zeng Y, Verschueren E, Kirkpatrick DS, Vucic D, Lee WP, Roose-Girma M, Newman RJ, Warming S, Hsiao YC, Komuves LG, Webster JD, Newton K, Dixit VM (2018) The deubiquitinating activity of OTULIN promotes targeted linear ubiquitination to limit cell death and inflammation. Nature 559:120–124
Hewitt JA, Brown LL, Murphy SJ, Grieder F, Silberberg SD (2017) Accelerating biomedical discoveries through rigor and transparency. ILAR J 58:115–128
Higashi AY, Ikawa T, Muramatsu M, Economides AN, Niwa A, Okuda T, Murphy AJ, Rojas J, Heike T, Tatsutoshi N, Kawamoto H, Kita T, Yanagita M (2009) Direct hematological toxicity and illegitimate chromosomal recombination caused by systemic activation of CreERT2. J Immunol 182:5633–5640
Huijbers IJ (2017) Generating genetically modified mice: a decision guide. In: Eroshenko N (ed) Methods in molecular biology, vol 1642. Site-specific recombinases: methods and protocols. Humana Press, New York, pp 1–19
IARC (International Agency for Research on Cancer)(n.d.) Agents classified by the IARC monographs, Volumes 1–123, online. Accessed on March 16, 2019. https://monographs.iarc.fr/wp-content/uploads/2019/02/List_of_Classifications.pdf
ICH (International Council on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use)—Safety (1997) Guidance for industry: S1B testing for carcinogenicity of pharmaceuticals. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S1B/Step4/S1B_Guideline.pdf. Accessed 16 Mar 2019
Jacobs AC, Brown PC (2015) Transgenic/alternative carcinogenicity assays: a retrospective review of studies submitted to CDER/FDA 1997-2014. Toxicol Pathol 43:605–610
Jacobs A, Jacobson-Kram D (2004) Human carcinogenic risk evaluation, part III: assessing cancer hazard and risk in human drug development. Toxicol Sci 81:260–262
Jacobson-Kram D, Sistare FD, Jacobs AC (2004) Use of transgenic mice in carcinogenicity hazard assessment. Toxicol Pathol 32:49–52
Janssen Biotech, Inc. (2016) STELARA® (ustekinumab) [Package insert]. Accessed on March 19, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761044lbl.pdf
Jinek M, Chylinksi K, Fonfara I, Haur M, Doudna JA, Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821
Junttila TT, Li J, Johnston J, Hristopoulos M, Clark R, Ellerman D, Wang B, Li Y, Mathieu M, Li G, Young J, Luis E, Phillips GL, Stefanich E, Spiess C, Polson A, Irving B, Scheer JM, Junttila MR, Dennis MS, Kelley R, Totpal K, Ebens A (2014) Antitumor efficacy of a bispecific antibody that targets HER2 and activates T cells. Cancer Res 74:5561–5571
Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C, Sundararajan A, Guo H, Roback L, Speck SH, Bertin J, Gough PJ, Balachandran S, Mocarski ES (2014) RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci U S A 111:7753–7758
Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P (1998) The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity 8:297–303
Kent WJ, Baertsch R, Hinrichs A, Miller W, Haussler D (2003) Evolution's cauldron: duplication, deletion, and rearrangement in the mouse and human genomes. Proc Natl Acad Sci U S A 100:11484–11489
Klein AD, Ferreira NS, Ben-Dor S, Duan J, Hardy J, Cox TM, Merrill AH Jr, Futerman AH (2016) Identification of modifier genes in a mouse model of Gaucher disease. Cell Rep 16:2546–2553
Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263–274
Kumar RT, Larson M, Wang H, McDermott J, Bronshteyn I (2009) Transgenic mouse technology: principles and methods. Methods Mol Biol 509:335–362
Lamb BT, Sisodia SS, Lawler AM, Slunt HH, Kitt CA, Kearns WG, Pearson PL, Price DL, Gearhart JD (1993) Introduction and expression of the 400 kilobase amyloid precursor protein gene in transgenic mice. Nat Genet 5:22–30
Lau J, Cheung J, Navarro A, Lianoglou S, Haley B, Totpal K, Sanders L, Koeppen H, Caplazi P, McBride J, Chiu H, Hong R, Grogan J, Javinal V, Yauch R, Irving B, Belvin M, Mellman I, Kim JM, Schmidt M (2017) Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat Commun 8:14572. https://doi.org/10.1038/ncomms14572
Lavelle GM, White MM, Browne N, McElvaney NG, Reeves EP (2016) Animal models of cystic fibrosis pathology: phenotypic parallels and divergences. Biomed Res Int 2016:5258727
Le Pichon CE, Meilandt WJ, Dominguez S, Solanoy H, Lin H, Ngu H, Gogineni A, Sengupta Ghosh A, Jiang Z, Lee SH, Maloney J, Gandham VD, Pozniak CD, Wang B, Lee S, Siu M, Patel S, Modrusan Z, Liu X, Rudhard Y, Baca M, Gustafson A, Kaminker J, Carano RAD, Huang EJ, Foreman O, Weimer R, Scearce-Levie K, Lewcock JW (2017) Loss of dual leucine zipper kinase signaling in protective in animal models of neurodegenerative disease. Sci Transl Med 9:eaag0394. https://doi.org/10.1126/scitranslmed.aag0394
Leder A, Kuo A, Cardiff RD, Sinn E, Leder P (1990) v-Ha-ras transgene abrogates the initiation step in mouse skin tumorigenesis: effects of phorbol esters and retinoic acid. Proc Natl Acad Sci U S A 87:9178–9182
Liljevald M, Rehnberg M, Söderberg M, Ramnegård M, Börjesson J, Luciani D, Krutrök N, Brändén L, Johansson C, Xu X, Bjursell M, Sjögren AK, Hornberg J, Andersson U, Keeling D, Jirholt J (2016) Retinoid-related orphan receptor γ (RORγ) adult induced knockout mice develop lymphoblastic lymphoma. Autoimmun Rev 15:1062–1070
Liu Y, Suckale J, Masjkur J, Magro MG, Steffen A, Anastassiadis K, Solimena M (2010) Tamoxifen-independent recombination in the RIP-CreER mouse. PLoS One 5:e13533
Lloyd KCK (2011) A knockout mouse resource for the biomedical research community. Ann N Y Acad Sci 1245:24–26
Madsen LW, Knauf JA, Gotfredsen C, Pilling A, Sjogren I, Andersen S, Anderson L, deBoer AS, Manova K, Barlaqs A, Vundavalli S, Nyborg NC, Knudsen LB, Moelck AM, Fagin JA (2012) GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology 153:1538–1547
Maeda A, Schneider SW, Kojima M, Beissert S, Schwarz T, Schwarz A (2006) Interleukin-12-deficient mice are at greater risk of UV radiation-induced skin tumors and malignant transformation of papillomas to carcinomas. Mol Cancer Ther 5:825–832
Manchado E, Huang CH, Tasdemir N, Tschaharganeh DF, Wilkinson JE, Lowe SW (2016) A pipeline for drug target identification and validation. Cold Spring Harb Symp Quant Biol 81:257–267
Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, Lich JD, Finger J, Kasparcova V, Votta B, Ouellette M, King BW, Wisnoski D, Lakdawala AS, DeMartion MP, Casillas LN, Haile PA, Sehon CA, Marquis RW, Upton J, Daley-Bauer LP, Roback L, Ramia N, Dovey CM, Carette JE, Ka-Ming Chan F, Bertin J, Gough PJ, Mocarski ES, Kaiser WJ (2014) RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell 56:481–495
Manning FJ, Swartz MN (1995) Review of the fialuridine (FIAU) clinical trials. National Academies Press, Washington (District of Columbia)
Martin P, Liu YN, Pierce R, Abou-Kheir W, Casey O, Seng V, Camacho D, Simpson RM, Kelly K (2011) Prostate epithelial Pten/TP53 loss leads to transformation of multipotential progenitors and epithelial to mesenchymal transition. Am J Pathol 179:422–435
Matthaei KI (2007) Genetically manipulated mice: a powerful tool with unsuspected caveats. J Physiol 582:481–488
May PC, Dean RA, Lowe SL, Martenyi F, Sheehan SM, Boggs LN, Monk SA, Mathes BM, Mergott DJ, Watson BM, Stout SL, Timm DE, Smith LaBell E, Gonzales CR, Nakano M, Jhee SS, Yen M, Ereshefsky L, Lindstrom TD, Calligaro DO, Cocke PJ, Greg Hall D, Friedrich S, Citron M, Audia JE (2011) Robust Central Reduction of Amyloid- in Humans with an Orally Available, Non-Peptidic -Secretase Inhibitor. J Neurosci 31(46):16507–16516
McCormick DL (2017) Chapter 12: preclinical evaluation of carcinogenicity using standard-bred and genetically engineered rodent models. In: Faqi AS (ed) A comprehensive guide to toxicology in nonclinical drug development, 2nd edn. Elsevier Academic Press, Cambridge, pp 273–292
McKenzie R, Fried MW, Sallie R, Conjeevaram H, Di Bisceglie AM, Park Y, Savarese B, Kleiner D, Tsokos M, Luciano C (1995) Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B. N Engl J Med 333:1099–1105
Meeran SM, Mantena SK, Meleth S, Elmets CA, Katiyar SK (2006) Enhanced photocarcinogenesis in interleukin-12-deficient mice. Cancer Res 66:2962–2969
Moggs JG, MacLachlan T, Martus HJ, Bentley P (2016) Derisking drug-induced carcinogenicity for novel therapeutics. Trends Cancer 2:398–408
Morton D, Alden CL, Bartholomew PM, Kreeger JM, Morton LD (2013) Volume II, chapter 27: carcinogenicity assessment. In: Haschek WM, Rousseaux CG, Wallig MA (eds) Haschek and Rousseaux’s handbook of toxicologic pathology, 3rd edn. Elsevier, pp 807–839
Morton D, Sistare FD, Nambiar PR, Turner OC, Radi Z, Bower N (2014) Regulatory forum commentary: alternative mouse models for future cancer risk assessment. Toxicol Pathol 42:799–806
Moulin M, Anderton H, Voss AK, Thomas T, Wei-Lynn W, Bankovacki A, Feltham R, Chau D, Cook WD, Silke J, Vaux DL (2012) IAPs limit activation of RIP kinases by TNF receptor 1 during development. EMBO 31:1679–1691
Newton K, Sun X, Dixit VM (2004) Kinase RIP3 is dispensible for normal NF-κBs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and toll-like receptors 2 and 4. Mol Cell Biol 24:1464–1469
Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D, Komuves L, Ferrando RE, Warming S, Dixit VM (2014) Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 343:1357–1360
Newton K, Dugger DL, Maltzmann A, Greve JM, Hedehus M, Martin-McNulty B, Carano RA, Cao TC, van Bruggen N, Bernstein L, Lee WP, Wu X, DeVoss J, Zhang J, Jeet S, Peng I, McKenzie BS, Roose-Girma M, Caplazi P, Diehl L, Webster JD, Vucic D (2016) RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ 23:1565–1576
Newton K, Dugger DL, Sengupta-Ghosh A, Ferrando RE, Chu F, Tao J, Lam W, Haller S, Chan S, Sa S, Dunlap D, Eastham-Anderson J, Ngu H, Hung J, French DM, Webster JD, Bolon B, Liu J, Reja R, Kummerfeld S, Chen YJ, Modrusan Z, Lewcock JW, Dixit VM (2018) Ubiquitin ligase COP1 coordinates transcriptional programs that control cell type specification in the developing mouse brain. Proc Natl Acad Sci U S A 115:11244–11249
Novo Nordisk (2009) Liraglutide (injection) for the treatment of patients with type 2 diabetes. NDA 22–341. http://citeseerx.ist.psu.edu/viewdoc/download;jsessionid=7095F8014984BF12976FF425BF30F962?doi=10.1.1.613.5008&rep=rep1&type=pdf. Accessed 17 Mar 2019
Okada S, Markle JG, Deenick EK, Mele F, Averbuch D, Lagos M, Alzahrani M, Al-Muhsen S, Halwani R, Ma CS, Wong N, Soudais C, Henderson LA, Marzouqa H, Shamma J, Gonzalez M, Martinez-Barricarte R, Okada C, Avery DT, Latorre D, Deswarte C, Jabot-Hanin F, Torrado E, Fountain J, Belkadi A, Itan Y, Boisson B, Migaud M, Arlehamn CSL, Sette A, Breton S, McCluskey J, Rossjohn J, de Villartay JP, Moshous D, Hambleton S, Latour S, Arkwright PD, Picard C, Lantz O, Engelhard D, Kobayashi M, Abel L, Cooper AM, Notarangelo LD, Boisson-Dupuis S, Puel A, Sallusto F, Bustamante J, Tangye SG, Casanova JL (2015) Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science 349:606–613
Olive KP, Tuveson DA (2006) The use of targeted mouse models for preclinical testing of novel cancer therapeutics. Clin Cancer Res 12:5277–5287
Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, DeNicola G, Feig C, Combs C, Winder SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Rückert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA (2009) Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324:1457–1461
Orban PC, Chui D, Marth JD (1992) Tissue- and site-specific DNA recombination in transgenic mice. Proc Natl Acad Sci U S A 89:6861–6865
Pelletier S, Gingras S, Green DR (2015) Mouse genome engineering via CRISPR-Cas9 for study of immune function. Immunity 42:18–26
Peltzer N, Darding M, Montinaro A, Draber P, Draberova H, Kupka S, Rieser E, Fisher A, Hutchinson C, Taraborrelli L, Hartwig T, Lafont E, Haas TL, Shimizu Y, Bӧiers C, Sarr A, Rickard J, Alvarez-Diaz S, Ashworth MT, Beal A, Enver T, Bertin J, Kaiser W, Strasser A, Silke J, Bouillet P, Walczak H (2018) LUBAC is essential for embryogenesis by preventing cell death and enabling haematopoiesis. Nature 557:112–117
Perleberg C, Kind A, Schnieke (2018) Genetically engineered pigs and models for human disease. Dis Model Mech 11(1). https://doi.org/10.1242/dmm.030783
Polykratis A, Hermance N, Zelic M, Roderick J, Kim C, Van TM, Lee TH, Chan FKM, Pasparakis M, Kelliher MA (2014) Cutting edge: RIPK1 kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J Immunol 193:1539–1543
Prinz F, Schlange T, Asadullah K (2011) Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov 10:712–713
Pritchard JB, French JE, Davis BJ, Haseman JK (2003) The role of transgenic mouse models in carcinogen identification. Environ Health Perspect 111:444–454
Rajapaksa KS, Huang T, Sharma N, Liu S, Solon M, Reyes A, Paul S, Yee A, Tao J, Chalasani S, Bien-Ly N, Barck K, Carano RA, Wang J, Rangell L, Bremer M, Danilenko DM, Katavolos P, Hotzel I, Reif K, Austin CD (2016) Preclinical safety profile of a depleting antibody against CRTh2 for asthma: well tolerated despite unexpected crth2 expression on vascular pericytes in the central nervous system and gastric mucosa. Toxicol Sci 152:72–84
Rosenthal N, Brown S (2007) The mouse ascending: perspectives for human-disease models. Nat Cell Biol 9:993–999
Saitoh A, Kimura M, Takahasi R, Yokoyama M, Nomura T, Izawa M, Sckiya T, Nishimura S, Katsuki M (1990) Most tumors in transgenic mice with human c-Ha-ras gene contained somatically activated transgenes. Oncogene 5:1195–1200
Sauer B, Henderson N (1988) Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci U S A 85:5166–5170
Schindowski K, Bretteville A, Leroy K, Bégard S, Brion JP, Hamdane M, Buée L (2006) Alzheimer's disease-like tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated tau transgenic mouse without any motor deficits. Am J Pathol 169:599–616
Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cushieri J, Bankey PE, Johnson JL, Speery J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG, the Inflammation and Host Response to Injury, Large Scale Collaborative Research Program (2013) Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 110:3507–3512
Sharpe ME, Morton D, Rossi A (2012) Nonclinical safety strategies for stem cell therapies. Toxicol Appl Pharmacol 262:223–231
Shen B, Zhang J, Wu H, Wang J, Ma K, Li Z, Zhang X, Zhang P, Huang X (2013) Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Res 23:720–723
Shive HR (2013) Zebrafish models for human cancer. Vet Pathol 50:468–482
Shultz LD, Ishikawa F, Greiner DL (2007) Humanized mice in translational biomedical research. Nature Rev Immunol 7:118–130
Shultz LD, Keck J, Burzenski L, Jangalwe S, Vaidya S, Greiner DL, Brehm MA (2019) Humanized mouse models of immunological diseases and precision medicine. Mamm Genome 30:123–142
Singh M, Lima A, Molina R, Hamilton P, Clermont AC, Devasthali V, Thompson JD, Cheng JH, Reslan HB, Ho CCK, Cao TC, Lee CV, Nannini MA, Fuh G, Carano RAD, Koeppen H, Yu RX, Forrest WF, Plowman GD, Johnson L (2010) Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nat Biotechnol 28:585–593
Soriano P (1999) Generalized lacz expression with the ROSA26 Cre reporter strain. Nature Genet 21:70–71
Spalding JW, French JE, Tice RR, Furedi-Machacek M, Haseman JK, Tennant RW (1999) Development of a transgenic mouse model for carcinogenesis bioassays: evaluation of chemically induced skin tumors in Tg.AC mice. Toxicol Sci 49:241–254
Spalding JW, French JE, Stasiewicz S, Furedi-Machacek M, Conner F, Tice RR, Tennant RW (2000) Responses of transgenic mouse lines p53(+/−) and Tg.AC to agents tested in conventional carcinogenicity bioassays. Toxicol Sci 53:213–223
Storer RD, French JE, Haseman J, Hajian G, LeGrand EK, Long GG, Mixson LA, Ochoa R, Sagartz JE, Soper KA (2001) P53+/− hemizygous knockout mouse: overview of available data. Toxicol Pathol 29:30–50
Storer RD, Sistare FD, Reddy MV, DeGeorge JJ (2010) An industry perspective on the utility of short-term carcinogenicity testing in transgenic mice in pharmaceutical development. Toxicol Pathol 38:51–61
Strom SC, Davila J, Grompe M (2010) Chimeric mice with humanized liver: tools for the study of drug metabolism, excretion, and toxicity. Methods Mol Biol 640:491–450
Sun Y, Peng I, Webster JD, Suto E, Lesch J, Wu X, Senger K, Francis G, Barrett K, Collier JL, Burch JD, Zhou M, Chen Y, Chan C, Eastham-Anderson J, Ngu H, Li O, Staton T, Havnar C, Jaochico A, Jackman J, Jeet S, Riol-Blanco L, Wu L, Choy DF, Arron JR, McKenzie BS, Ghilardi N, Hicham Alaoui Ismaili M, Pei Z, DeVoss J, Austin CD, Lee WP, Zarrin AA (2015) Inhibition of the kinase ITK in a mouse model of asthma reduces cell death and fails to inhibit the inflammatory response. Sci Signal 8:ra122. https://doi.org/10.1126/scisignal.aab0949
Swedish Orphan Biovitrum AB (2006) Initial marketing-authorisation documents Kineret: EPAR—scientific discussion. EMEA European Medicines Agency. Accessed on March 19, 2019. https://www.ema.europa.eu/en/documents/scientific-discussion/kineret-epar-scientific-discussion_en.pdf
Takao K, Miyakawa T (2015) Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 112:1167–1172
Tennant RW, Stasiewicz S, Eastin WC, Mennear JH, Spalding JW (2001) The Tg. AC (v-Ha-ras) transgenic mouse: nature of the model. Toxicol Pathol 29:51–59
Thomas DW, Burns J, Audette J, Carroll A, Dow-Hygelund C, Hay M (2016) Clinical development success rates 2006–2015. BIO Industry Analysis. https://www.bio.org/bio-industry-analysis-reports
Ueda E, Kurebayashi S, Sakaue M, Backlund M, Koller B, Jetten AM (2002) High incidence of T-cell lymphomas in mice deficient in the retinoid-related orphan receptor ROR gamma. Cancer Res 62:901–909
Vahle JL, Finch GL, Heidel SM, Hovland DN Jr, Ivens I, Parker S, Ponce RA, Sachs C, Steigerwalt R, Short B, Todd MD (2010) Carcinogenicity assessments of biotechnology-derived pharmaceuticals: a review of approved molecules and best practice recommendations. Toxicol Pathol 38:522–553
Van Zeller AM, Combes RD (1999) Transgenic mouse bioassays for carcinogenicity testing: a step in the right direction? ATLA 27:839–846
Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann VM, Kemper OC, Kollet O, Lapidot T, Soffer D, Sobe T, Avraham KB, Goncharov T, Holtmann H, Lonai P, Wallach D (1998) Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9:267–276
Wang H, Yang H, Sivialila CS, Dawlaty MM, Cheng AW, Zhang F, Jaensich R (2013) One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153:910–918
Wilke M, Buijs-Offerman RM, Aarbiou J, Colledge WH, Sheppard DN, Touqui L, Bot A, Jorna H, de Jonge HR, Scholte BJ (2011) Mouse models of cystic fibrosis: phenotypic analysis and research applications. J Cyst Fibros 10(Suppl 2):S152–S171
Wittkopf N, Gunther C, Martini E, He G, Amann K, He YW, Schuchmann M, Neurath MF, Becker C (2013) Cellular FLIC-like inhibitory protein secures intestinal epithelial cell survival and immune homeostasis by regulating caspase 8. Gastroenterol 45:1396–1379
Xu YH, Quinn B, Witte D, Grabowski GA (2003) Viable mouse models of acid beta-glucosidase deficiency: the defect in Gaucher disease. Am J Pathol 163:2093–2101
Xu D, Nishimura T, Nishimura S, Zhang H, Zheng M, Guo YY, Masek M, Michie SA, Glenn J, Peltz G (2014) Fialuridine induces acute liver failure in chimeric TK-NOG mice: a model for detecting hepatic drug toxicity prior to human testing. PLoS Med 11:e1001628
Yamamoto S, Mitsumori K, Kodama Y, Matsunuma N, Manabe S, Okamiya H, Suzuki H, Fukuda T, Sakamaki Y, Sunaga M, Nomura G, Hioki K, Wakana S, Nomura T, Hayashi Y (1996) Rapid induction of more malignant tumors by various genotoxic carcinogens in transgenic mice harboring a human prototype c-Ha-ras gene than in control non-transgenic mice. Carcinogenesis 17:2455–2461
Yamamoto S, Urano K, Koizumi H, Wakana S, Hioki K, Mitsumori K, Kurokawa Y, Hayashi Y, Nomura T (1998) Validation of transgenic mice carrying the human prototype c-Ha-ras gene as a bioassay model for rapid carcinogenicity testing. Environ Health Perspect 106:57–69
Yang H, Wu Z (2018) Genome editing of pigs for agriculture and biomedicine. Front Genet 9:360. https://doi.org/10.3389/fgene.2018.00360
Yeh WC, Itie A, Elia AJ, Ng M, Shu HB, Wakeham A, Mirtsos C, Suzuki N, Bonnard M, Goeddel DV, Mak TW (2000) Requirement for casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity 12:633–642
Yoshimi K, Mashimo T (2018) Application of genome editing technologies in rats for human disease models. J Hum Genet 63:115–123
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors are employees of Genentech and, as such, stockholders in Roche. The authors have no other conflicts of interest or funding sources to report.
Human and animal rights and informed consent
As a review article, this paper does not contain any original studies with human participants or animals performed by any of the authors. Some referenced published data includes animal studies performed by the authors. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Webster, J.D., Santagostino, S.F. & Foreman, O. Applications and considerations for the use of genetically engineered mouse models in drug development. Cell Tissue Res 380, 325–340 (2020). https://doi.org/10.1007/s00441-019-03101-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-019-03101-y