Abstract
Purpose
Keratin 17 (KRT17) has been suggested as a potential diagnostic marker of squamous cell carcinoma including oral squamous cell carcinoma (OSCC). The current study was conducted to clarify the function of KRT17 and its expression mechanism in OSCC.
Methods
Immunohistochemical analyses were carried out to examine the expression of KRT17, GLI family zinc finger (GLI)-1, GLI-2, or cleaved caspase-3 in OSCCs. The expression of KRT17, GLI-1, or GLI-2 was investigated among OSCC cell lines, and the effects of loss-of-function of KRT17 or GLI, using siRNA or inhibitor, on the cell growth of the OSCC cell line HSC-2 particularly with respect to apoptosis were examined.
Results
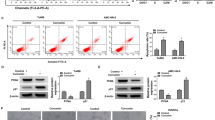
Immunohistochemical analyses of tissue specimens obtained from 78 OSCC patients revealed that KRT17 was not observed in non-tumor regions but was strongly expressed at high frequencies in tumor regions. Knockdown of KRT17 increased the number of cleaved caspase-3-positive cells, leading to the reduction of cell number. Loss-of-function of GLI-1 or GLI-2 also increased the cell numbers of apoptotic cells positive for staining of Annexin-V and propidium iodide (PI) and the terminal deoxynucleotidyl transferase dUTP-biotin nick-end labeling (TUNEL) method, and induced DNA fragmentation. This inhibitory effect on cell growth was partially rescued by exogenous KRT17 expression. In the KRT17-positive regions in OSCCs, GLI-1 or GLI-2 was frequently detected, and the number of cells with cleaved caspase-3 positive was decreased.
Conclusions
KRT17 promotes tumor cell growth, at least partially, through its anti-apoptotic effect as a result of the KRT17 overexpression by GLIs in OSCC.






Similar content being viewed by others
References
Atale N, Gupta S, Yadav UC, Rani V (2014) Cell-death assessment by fluorescent and nonfluorescent cytosolic and nuclear staining techniques. J Microsc 255:7–19
Barnes L, Eveson JW, Reichart P, Sidransky D (eds) (2005) Pathology and Genetics of Head and Neck Tumours, 3rd edn. IARC, France
Bianchi N, Depianto D, Mcgowan K, Gu C, Coulombe PA (2005) Exploiting the Keratin 17 gene promoter to visualize live cells in epithelial appendages of mice. Mol Cell Biol 25:7249–7259
Bortoluzzi MC, Yurgel LS, Dekker NP, Jordan RC, Regezi JA (2004) Assessment of p63 expression in oral squamous cell carcinomas and dysplasias. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 98:698–704
Carrilho C, Alberto M, Buane L, David L (2004) Keratins 8, 10, 13, and 17 are useful markers in the diagnosis of human cervix carcinomas. Hum Pathol 35:546–551
Chidzonga MM (2006) Oral malignant neoplasia: a survey of 428 cases in two Zimbabwean hospitals. Oral Oncol 42:177–183
Chidzonga MM, Mahomva L (2006) Squamous cell carcinoma of the oral cavity, maxillary antrum and lip in a Zimbabwean population: a descriptive epidemiological study. Oral Oncol 42:184–189
Cohen-Kerem R, Madah W, Sabo E, Rahat MA, Greenberg E, Elmalah I (2004) Cytokeratin-17 as a potential marker for squamous cell carcinoma of the larynx. Ann Otol Rhinol Laryngol 113:821–827
Dimery IW, Hong WK (1993) Overview of combined modality therapies for head and neck cancer. J Natl Cancer Inst 85:95–111
Fujii S, Matsumoto S, Nojima S, Morii E, Kikuchi A (2015) Arl4c expression in colorectal and lung cancers promotes tumorigenesis and may represent a novel therapeutic target. Oncogene 34:4834–4844
Fujii S, Shinjo K, Matsumoto S, Harada T, Nojima S, Sato S et al (2016) Epigenetic upregulation of ARL4C, due to DNA hypomethylation in the 3′-untranslated region, promotes tumorigenesis of lung squamous cell carcinoma. Oncotarget 7:81571–81587
Gonnissen A, Isebaert S, Mckee CM, Dok R, Haustermans K, Muschel RJ (2016) The hedgehog inhibitor GANT61 sensitizes prostate cancer cells to ionizing radiation both in vitro and in vivo. Oncotarget 7:84286–84298
Honami T, Shimo T, Okui T, Kurio N, Hassan NM, Iwamoto M et al (2012) Sonic hedgehog signaling promotes growth of oral squamous cell carcinoma cells associated with bone destruction. Oral Oncol 48:49–55
Huang L, Walter V, Hayes DN, Onaitis M (2014) Hedgehog-GLI signaling inhibition suppresses tumor growth in squamous lung cancer. Clin Cancer Res 20:1566–1575
Ikeda K, Tate G, Suzuki T, Mitsuya T (2008) Coordinate expression of cytokeratin 8 and cytokeratin 17 immunohistochemical staining in cervical intraepithelial neoplasia and cervical squamous cell carcinoma: an immunohistochemical analysis and review of the literature. Gynecol Oncol 108:598–602
Khanom R, Nguyen CT, Kayamori K, Zhao X, Morita K, Miki Y et al (2016) Keratin 17 is induced in oral cancer and facilitates tumor growth. PLoS One 11:e0161163
Kim S, Wong P, Coulombe PA (2006) A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature 441:362–365
Kitamura R, Toyoshima T, Tanaka H, Kawano S, Kiyosue T, Matsubara R et al (2012) Association of cytokeratin 17 expression with differentiation in oral squamous cell carcinoma. J Cancer Res Clin Oncol 138:1299–1310
Kiyoshima T, Yoshida H, Wada H, Nagata K, Fujiwara H, Kihara M et al (2013) Chemoresistance to concanamycin A1 in human oral squamous cell carcinoma is attenuated by an HDAC inhibitor partly via suppression of Bcl-2 expression. PLoS One 8:e80998
Kurokawa H, Yamashita Y, Tokudome S, Kajiyama M (1997) Combination assay for tumor markers in oral squamous cell carcinoma. J Oral Maxillofac Surg 55:964–966
Llewellyn CD, Linklater K, Bell J, Johnson NW, Warnakulasuriya KA (2003) Squamous cell carcinoma of the oral cavity in patients aged 45 years and under: a descriptive analysis of 116 cases diagnosed in the South East of England from 1990 to 1997. Oral Oncol 39:106–114
Matsuo K, Ishibashi Y, Kobayashi I, Ozeki S, Ohishi M, Tange T et al (1994) New human oral squamous carcinoma cell line and its tumorigenic subline producing granulocyte colony-stimulating factor. Jpn J Cancer Res 85:1257–1262
McGowan KM, Coulombe PA (1998) Onset of keratin 17 expression coincides with the definition of major epithelial lineages during skin development. J Cell Biol 143:469–486
Mikami T, Cheng J, Maruyama S, Kobayashi T, Funayama A, Yamazaki M et al (2011) Emergence of keratin 17 vs. loss of keratin 13: their reciprocal immunohistochemical profiles in oral carcinoma in situ. Oral Oncol 47:497–503
Mikami T, Maruyama S, Abé T, Kobayashi T, Yamazaki M, Funayama A et al (2015) Keratin 17 is co-expressed with 14-3-3 sigma in oral carcinoma in situ and squamous cell carcinoma and modulates cell proliferation and size but not cell migration. Virchows Arch 466:559–569
Miyoshi H, Blömer U, Takahashi M, Gage FH, Verma IM (1998) Development of a self-inactivating lentivirus vector. J Virol 72:8150–8157
Morifuji M, Taniguchi S, Sakai H, Nakabeppu Y, Ohishi M (2000) Differential expression of cytokeratin after orthotopic implantation of newly established human tongue cancer cell lines of defined metastatic ability. Am J Pathol 156:1317–1326
Nieto MA (2013) Epithelial plasticity: a common theme in embryonic and cancer cells. Sci 342:1234850
Nybakken K, Perrimon N (2002) Hedgehog signal transduction: recent findings. Curr Opin Genet Dev 12:503–511
Pan X, Hobbs RP, Coulombe PA (2013) The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr Opin Cell Biol 25:47–56
Pasca di Magliano M, Hebrok M (2003) Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer 3:903–911
Ruiz i Altaba A, Sánchez P, Dahmane N (2002) Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer 2:361–372
Sankar S, Tanner JM, Bell R, Chaturvedi A, Randall RL, Beckerle MC et al (2013) A novel role for keratin 17 in coordinating oncogenic transformation and cellular adhesion in Ewing sarcoma. Mol Cell Biol 33:4448–4460
Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H (1999) Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development 17:3915–3924
Schantz SP, Yu GP (2002) Head and neck cancer incidence trends in young Americans, 1973–1997, with a special analysis for tongue cancer. Arch Otolaryngol Head Neck Surg 128:268–274
Sobin LH, Gospodarowicz MK, Wittekind C (eds) (2009) TNM classification of malignant tumors, 7th edn. UICC, Switzerland
Sun Y, Guo W, Ren T, Liang W, Zhou W, Lu Q et al (2014) Gli1 inhibition suppressed cell growth and cell cycle progression and induced apoptosis as well as autophagy depending on ERK1/2 activity in human chondrosarcoma cells. Cell Death Dis 5:e979
Takahashi H, Shikata N, Senzaki H, Shintaku M, Tsubura A (1995) Immunohistochemical staining patterns of keratins in normal oesophageal epithelium and carcinoma of the oesophagus. Histopathology 26:45–50
Toyoshima T, Vairaktaris E, Nkenke E, Schlegel KA, Neukam FW, Ries J (2008) Cytokeratin 17 mRNA expression has potential for diagnostic marker of oral squamous cell carcinoma. J Cancer Res Clin Oncol 134:515–521
van de Rijn M, Perou CM, Tibshirani R, Haas P, Kallioniemi O, Kononen J et al (2002) Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol 161:1991–1996
Wang MT, Holderfield M, Galeas J, Delrosario R, To MD, Balmain A et al (2015) K-Ras promotes tumorigenicity through suppression of non-canonical Wnt signaling. Cell 163:1237–1251
Yamamoto E, Miyakawa A, Kohama G (1984) Mode of invasion and lymph node metastasis in squamous cell carcinoma of the oral cavity. Head Neck Surg 6:938–947
Yan M, Wang L, Zuo H, Zhang Z, Chen W, Mao L et al (2011) HH/GLI signalling as a new therapeutic target for patients with oral squamous cell carcinoma. Oral Oncol 47:504–509
Acknowledgements
The authors thank Dr. R. Kitamura for valuable help regarding statistical analyses and Dr. H. Fujiwara for assistance with the cell death assay and DNA fragmentation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Ethical approval for this study was obtained from the institutional review board of Kyushu University Hospital.
Informed consent
Written informed consent was obtained from individual participants.
Funding
This work was supported by Grants-in-Aid for Scientific Research to K. T. (No. 15K15695), S. F. (No. 16K11501), and H. W. (No. 26462788) from the Ministry of Education, Science, and Culture of Japan.
Additional information
Y. Mikami and S. Fujii have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mikami, Y., Fujii, S., Nagata, K. et al. GLI-mediated Keratin 17 expression promotes tumor cell growth through the anti-apoptotic function in oral squamous cell carcinomas. J Cancer Res Clin Oncol 143, 1381–1393 (2017). https://doi.org/10.1007/s00432-017-2398-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-017-2398-2