Abstract
Purpose
Long-term administration of adjuvant temozolomide is common practice in many institutions, especially when treatment is well tolerated and stable disease is achieved. In this study, we evaluate the feasibility and efficacy of long-term temozolomide in patients with glioblastoma multiforme treated at a single institution.
Methods
One hundred and fourteen patients with newly diagnosed glioblastoma were followed for the course of their disease. Treatment consisted of surgery [gross total resection (GTR) subtotal resection (STR) or biopsy] followed by radiotherapy and concomitant temozolomide. Adjuvant temozolomide was administered until evidence for progressive disease or serious side effects occurred. Follow-up was routinely performed every 3 months.
Results
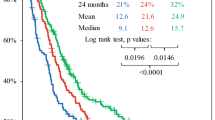
One hundred and fourteen patients with glioblastoma multiforme received a median of 6 cycles of adjuvant first-line temozolomide (range 1–57). For patients with less than 6 cycles, chemotherapy was stopped in 60% for reasons other than progression, while only in 17% of patients receiving 6 or more (P < 0.0001). Median TTP was 7 months (95% CI: 6–10 months). PFS after 6 months was 53%. Median OS in all patients was 15 months (95% CI: 13–18 months). TTP and OS directly correlate with the amount of chemotherapy cycles (each P < 0.0001). No significant influence of the extent of surgical treatment on PFS (P = 0.2141) and OS (P = 0.4308) could be detected.
Conclusion
This data set suggests that long-term administration of temozolomide is safe and efficacious. Side effects occur more frequently in the early phase of drug administration (<6 cycles). There is a strong correlation of long-term temozolomide on PFS and OS regardless of the extent of surgery and other factors.


Similar content being viewed by others
References
DeAngelis LM (2001) Brain Tumours. N Engl J Med 344:114–123
Hau P, Koch D, Hundsberger T et al (2007) Safety and feasibility of long-term temozolomide treatment in patients with high-grade glioma. Neurology 68:688–690
Khasraw M, Bell D, Wheeler H (2009) Long-term use of temozolomide: could you use temozolomide safely for life in gliomas? J Clin Neurosci 16:854–855
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 35:978–996
Trotti A, Colevas AD, Setser A et al (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13:176–181
Conflict of interest statement
None
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Seiz and U. Krafft contributed equally to this study.
Rights and permissions
About this article
Cite this article
Seiz, M., Krafft, U., Freyschlag, C.F. et al. Long-term adjuvant administration of temozolomide in patients with glioblastoma multiforme: experience of a single institution. J Cancer Res Clin Oncol 136, 1691–1695 (2010). https://doi.org/10.1007/s00432-010-0827-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-010-0827-6