Abstract
Cephalohematoma is a common pathology in newborns. Observation is the primary treatment for most patients with small uncomplicated cephalohematoma. Conversely, a large cephalohematoma can lead to calcification with unesthetic local deformation or deformational plagiocephaly. The objective of the study was to evaluate the iatrogenic risk associated with early puncture under local anesthesia and oral sucrose. This is a retrospective study of 67 consecutive newborns followed at Montpellier University Hospital, France, between 2010 and 2017. Large cephalohematoma was defined on the basis of the bump projection. Due to the uncertainty of the spontaneous resorption and the risk of calcification after 4 weeks which render the needle aspiration ineffective, puncture was performed between 2 and 4 weeks of life after coagulation evaluation and ultrasound of the skull and scalp. Puncture was performed in 43 boys (64%) and 24 (36%) girls between day 15 and day 30 after birth. The cephalohematoma maximal projection measured by ultrasound ranged from 9 to 13 mm (Q1,Q4) with a median value of 12 mm. No puncture-related complication was recorded during the intervention and at the 1-month follow-up visit.
Conclusion: In newborns with large and persistent unesthetic cephalohematoma, puncture under local anesthesia with oral sucrose can be safely proposed between day 15 and day 30 after birth.
What is Known: • Infant cephalohematoma is a frequent pathology of newborns, consisting of a traumatic subperiosteal hematoma of the skull. Most cephalohematomas are small and require no treatment because they spontaneously disappear within the first month. • Large and non-resorptive cephalohematomas may have significant esthetic and functional consequences. What is New: • Early puncture under local anesthesia is a safe, effective, and rapid procedure, decreasing the risk of persistent skull deformities. • Puncture can be proposed for newborns with a large (high projection and/or high angle connection) persistent anesthetic cephalohematoma, between day 15 and day 30, before spontaneous calcification. |
Similar content being viewed by others
Introduction
Infant cephalohematoma occurs in 0.5 to 2% of newborns [1,2,3]. It is a traumatic subperiosteal hematoma of the skull bone and must be differentiated from subgaleal hematoma and caput succedaneum [4]. At physical examination, cephalohematoma is characterized by a bulge/swelling on the infant’s head due to blood accumulation between periosteum and skull, and is usually delimited by the suture lines due to the periosteum attachment to the bone’s edge. Cephalohematoma physiopathology is poorly understood, but it is considered to be caused by bleeding of the emissary or diploic veins after the detachment of the subperiosteal layer of the skull. This slow bleeding can lead to an increase in the size during the first days of life before it spontaneously stops limited by the sutures. The risk factors are compression and shear strain to the scalp, in utero [5] or during labor and delivery. Instrument-assisted delivery, prolonged labor, large infants, and uncommon head presentation are classically associated with cephalohematoma [3]. For unknown reason is more frequent in newborn boys. Cephalohematoma is mostly located over the parietal bone and on the right side, and is bilateral in 10% of cases. It can be associated with linear skull fractures [6, 7], and in this case, it requires specific pediatric neurosurgery treatment. Infection is a rare complication, after systemic infection or focal skin lesion [8, 9]. Large cephalohematoma can induce hyperbilurubinemia in newborns [10, 11], or anemia [12].
Most cephalohematomas are small and require no treatment because they spontaneously disappear within the first month for most of them [13]. As cephalohematoma management is mainly observational, primary healthcare professionals play a significant role in providing reassurance to the new parents who are often very anxious [14]. On rare occasions, cephalohematomas persist beyond 4 weeks. Progressive calcification can be observed in these cephalohematomas, leading to a small bump on the calvaria that should disappear during the skull remodeling following its rapid growth in the first year of life [15,16,17]. However, cephalohematoma should be monitored until complete resorption. Incomplete resorption of large cephalohematoma can lead to persistent unesthetic deformation of the calvaria. Indeed, the bump on the side of the cephalhematoma may causes contro-lateral occipital and frontal molding and cervical rotation leading to deformational plagiocephaly [18].
In the case of large and non-resorptive cephalhematoma, needle aspiration may be an alternative to prevent progression of this molding [19].
Firlik and Adelson [18] proposed in 1999 needle puncture for persistent cephalohematoma. Although aspiration of a cephalohematoma is technically uncomplicated, improper technique may predispose the patient to a scalp infection or osteomyelitis [8, 9].
No study is available in the literature about the outcomes of this procedure. In our Pediatric Orthopedic Plastic Surgery Unit, to avoid the risk of incomplete resorption, we propose puncture under local anesthesia to selected patients with large or non-resorptive cephalohematoma. The objective of this study was to retrospectively evaluate the iatrogenic risk associated with this procedure under local anesthesia.
Materials and methods
This is a retrospective single-center observational study of infants with cephalohematoma consecutively followed at the Pediatric Orthopedic Plastic Surgery Unit, Montpellier University Hospital, France, and who underwent puncture under local anesthesia between 2010 and 2017. Patients with cephalohematoma who did not undergo puncture as well as infants with cephalohematoma associated with a neurosurgical pathology (n = 5) or with sepsis (n = 1; not linked to puncturing) were excluded from the study.
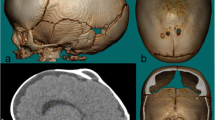
The diagnosis of cephalohematoma was performed by two senior plastic pediatric surgeons (G.C. and M.B.). Puncture was proposed to the parents in the case of large cephalohematoma with a heavily projected bump or with a very marked angle of connection between bump and scalp (Fig. 1). Only infants without any clear sign of resorption after 2 weeks of life underwent this intervention.
When puncture was indicated, blood coagulation and complete blood count were systematically determined before the procedure. Trans-fontanel ultrasonography was carried out to exclude any endocranial complication, and scalp/skull ultrasonography to exclude a fracture, confirm the diagnosis, and measure the height of the cephalohematoma between the skull and the periosteum. Cephalohematoma aspiration was performed after informing the parents about the risks associated with the procedure.
Puncture was carried out under not-fasting local anesthesia. Topical lidocaine cream 5% was applied 2 h before the puncture, followed by subcutaneous infiltration of lidocaine hydrochloride 5 mg/ml. Simultaneously, oral sucrose was also administered by a nurse [20]. The puncture was made on a quiet child and the nurse remains on the side of the patient’s face to assess if the procedure was comfortable the whole time.
Puncture was performed in the operating room using strict sterile techniques. The 20G spinal needle was introduced tangentially in the bump from the back side (Fig. 2), and blood was aspirated with a 20 cc syringe until there was no more blood and the bump had disappeared. The total volume of the collected blood was noted. No drain was put in place, and surgical dressing was left in place for 24 h. The infant was discharged home the same day without any anesthetic monitoring. Parent could wash their child’s head after 48 h.
Children were seen after 1 month to confirm the total cephalohematoma resorption and to provide preventive advice on deformational plagiocephaly [18].
Any potential complication during the month post-puncture was identified retrospectively during this study by analyzing the infants’ electronic medical record (peri-operative and post-operative reports, any visit to the Pediatric Emergency department, and the 1-month post-puncture clinical report).
The study had local review board approval from the Montpellier University Hospital research ethics committee.
Results
In total, 67 consecutive infants (43 boys, 64%, and 24 girls, 36%) underwent puncture between day 15 and day 30 after birth; 52 (78%) infants had unilateral cephalohematoma and 15 (22%) bilateral parietal cephalohematoma.
The maximal cephalohematoma height measured by ultrasound examination was available for 33 patients (49%); the median value was 12 mm (9;13) (1st and 3rd quartile). The total volume drained by needle aspiration was available for 30 patients (45%); the median value was 25 ml (16;34) (Fig. 3). The median blood volume for the 15 patients (22%) with bilateral parietal cephalohematoma was 23 ml (17;47). Blood volume and cephalohematoma height were significantly correlated (Pearson’s coefficient = 0.60, 95% CI [0.19–0.83], p = 0.008); however, the broad CI value does not support the clinical interest of this analysis.
No unexpected bleeding occurred during or after the puncture, and no cephalohematoma recurrence was reported after aspiration.
During the first month post-aspiration, two children went to the Pediatric Emergency Department for (i) afebrile skin rash 15 days after the intervention without any link with the previous procedure and (ii) fever at day 20 post-puncture. This child was kept under observation during the night, but no fever was detected at the hospital and he was discharged without treatment. The 1-month control report was available for 59 patients (88%) and did not record any complication.
Discussion
Cephalohematoma is caused by minor trauma of the head that often occur during/after birth. In the absence of resorption or in the case of large cephalohematoma, this condition may induce scalp deformations that require major surgery. Our study shows that in these cases, early puncture, between 15 and 30 days after birth under local anesthesia in the operating room, is a safe procedure. No complication was reported during the puncture or in the following month. In each case, the cephalohematoma was cured and no follow-up was necessary after one month.
To our knowledge, this is the first study that describes the puncture of cephalohematoma. There is no consensus about the management of non-spontaneously resorbing large cephalohematoma of newborns. For many authors [2, 4, 21], observation is the best strategy, arguing that spontaneously favorable evolution in the long term is frequent. However, Firlik and Adelson [18] proposed in 1999 a protocol to avoid delayed scalp deformity and delayed open surgical interventions. They recommended puncture for all patients with cephalohematoma after 1 month of observation. In our mind, puncture has to be done before 1 month to avoid difficult evacuation of the hematoma due to ossification.
Eseonu et al. [22] reported in 2016 the first case of surgical evacuation of a large cephalohematoma by small scalp incision. They left a drain for 24 h after the procedure. This type of drainage is very efficient and fully evacuates the blood within the hematoma. Nevertheless, it requires general anesthesia with the associated risks in newborns [23] and several organizational constraints: consultation with the anesthesiologist, empty stomach, and post-surgery follow-up. In our opinion, this kind of protocol is too binding to be used in the routine clinical practice. Our method of puncture under local anesthesia can be done in an outpatient setting, if the condition of strict asepsis is fulfilled.
A Cochrane meta-analysis [24] showed that buffered lidocaine can considerably minimize pain. We could in the future consider buffered lidocaine (10:1 lidocaine:bicarbonate 8.4%) in order to make the injection of the local anesthetic completely pain-free.
A limitation of the study is the absence of objective criteria leading to the puncture indication. Eseonu et al. [22] proposed a cut-off of 7 cm for the bigger dimension to identify large cephalohematomas that require drainage. However, smaller cephalohematoma with a high projection above the scalp and with a sharp angle of connection between the bump and the scalp may have a major esthetic and functional impact. In our study, puncture was proposed by the two senior surgeons on clinical examination. Ultrasound examination was done after this proposition to measure the bump projection. In practice, 73% (n = 24) of patients selected by the surgeons had a cephalohematoma with a bump projection higher than 9 mm, which could be considered as a cut-off to help decision-making. Nevertheless, the most relevant criterion seems to be a cephalohematoma with a strong esthetic impact. This subjective evaluation has to take into account also the parents’ expectations. Scalp/skull and trans-fontanel ultrasound imaging by an experienced radiologist seems to be more pertinent than skull X-ray because of the absence of radiation. It informs about the association with skull fracture or intracranial hematoma.
The major puncture risk is infection that can lead to local abscess, osteomyelitis of the skull, or meningitis [22]. This risk is controlled by the strict sterile condition of the operating room. We think that a post-operative drain is not required because of the efficacy of the early puncture (before one month of life). In agreement, Eseonu et al. [22] reported that only 2 cc of blood was drained after 24 h. Moreover, leaving a drain in the hematoma can increase the risk of infectious events.
The risk of hemorrhage exists if puncturing is performed when venous bleeding is still ongoing. For this reason, it is important to wait at least 2 weeks after birth when bleeding should have spontaneously stopped.
Avoiding local deformation and deformational plagiocephaly [18] is the major argument in favor of early puncture of a large anesthetic cephalohematoma to decrease the risk of persistent skull deformities and the need of open skull surgery [25, 26].
Conclusion
Puncture of large anesthetic cephalohematoma under local anesthesia in the operating room between 2 weeks and 1 month after birth seems to be a safe and effective procedure. The puncture is done after skull ultrasound and coagulation assessment. Puncture may be proposed for newborns with a large (high projection and/or high angle connection) persistent anesthetic cephalohematoma to avoid skull deformities.
References
Thacker KE, Lim T, Drew JH (1987) Cephalhaematoma: a 10-year review. Aust N Z J Obstet Gynaecol 27(3):210
Yasunaga S, Rivera R (1974) Cephalhematoma in the newborn. Clin Pediatr (Phila) 13(3):256–260
Mangurten HH (2006) Birth injuries. In: Martin RJ, Fanaroff AA, Walsh MC (eds) Neonatal-perinatal medicine: diseases of the fetus and infant. Philadelphia, PA :Elsevier/Saunders, pp 531–535
Nicholson L (2007) Caput succedaneum and cephalohematoma: the cs that leave bumps on the head. Neonatal Netw 26(5):277–281
Petrikovsky BM, Schneider E, Smith-Levitin M, Gross B (1998) Cephalhematoma and caput succedaneum: do they always occur in labor? Am J Obstet Gynecol 179(4):906–908
Zakanj Z (2014) Skull fracture and cephalhematoma in a newborn-a case report. Lijec Vjesn 136(11-12):335–338
Kendall N, Woloshin H (1952) Cephalhematoma associated with fracture of the skull. J Pediatr 41(2):125–132
Blom NA, Vreede WB (1993) Infected cephalhematomas associated with osteomyelitis, sepsis and meningitis. Pediatr Infect Dis J 12(12):1015–1017
Wong CS, Cheah FC (2012) Cephalhematoma infected by Escherichia coli presenting as an extensive scalp abscess. J Pediatr Surg 47(12):2336–2340
Bansal A, Bothra GC, Verma CR (1985) Hyperbilirubinemia due to massive cephalhematoma. Indian Pediatr 22(8):619–621
Balmes J, Pujol H (1953) Cephalhematoma of the newborn and maternal and fetal thrombocytopenia. Montpellier Med 44(4):282–286
Leonard S, Anthony B (1961) Giant cephalhematoma of newborn: with hemorrhagic disease and hyperbilirubinemia. Am J Dis Children 101(2):170–173
Ciurea AV, Gorgan MR, Tascu A, Sandu AM, Rizea RE (2011) Traumatic brain injury in infants and toddlers, 0–3 years old. J Med Life 4(3):234–243
Paul SP, Goodman A (2011) Potential complications of neonatal cephalhaematoma in the community: when to refer to the paediatric team? J Fam Health Care 21(1):16–19
Daglioglu E, Okay O, Hatipoglu HG, Dalgic A, Ergungor F (2010) Spontaneous resolution of calcified cephalhematomas of infancy: report of two cases. Turk Neurosurg 20(1):96–99
Guclu B, Yalcinkaya U, Kazanci B, Adilay U, Ekici MA (2012) Diagnosis and treatment of ossified cephalhematoma. J Craniofac Surg 23(5):e505–e507
Yoon SD, Cho BM, Oh SM, Park SH (2013) Spontaneous resorption of calcified cephalhematoma in a 9-month-old child: case report. Childs Nerv Syst 29(3):517–519
Firlik KS, Adelson PD (1999) Large chronic cephalohematoma without calcification. Pediatr Neurosurg 30(1):39–42
Gupta PK, Mathew GS, Malik AK, Al Derazi T (2007) Ossified cephalhematoma. Pediatr Neurosurg 43(6):492–497
Matsuda E (2017) Sucrose as analgesia in neonates undergoing painful procedures. Am J Nurs 117(8):21
Unrein H (1970) Conservative or surgical treatment of cephalhematoma. Kinderarztl Prax 38(12):552
Eseonu CI, Sacino AN, Ahn ES (2016) Early surgical intervention for a large newborn cephalohematoma. Pediatr Neurosurg 51(4):210–213
Brockel MA, Polaner DM, Vemulakonda VM (2018) Anesthesia in the pediatric patient. Urol Clinic N Am 45(4):551–560
Cepeda M, Tzortzopoulou A, Thackrey M et al (2010) Adjusting the pH of lidocaine for reducing pain on injection. Cochrane Database Syst Rev 12:CD006581
Chung HY, Chung JY, Lee DG, Yang JD, Baik BS, Hwang SG, Cho BC (2004) Surgical treatment of ossified cephalhematoma. J Craniofac Surg 15(5):774–779
Liu L, Dong C, Chen L (2016) Surgical treatment of ossified cephalhematoma: a case report and review of the literature. World Neurosurg 96:614.e7–614.e9
Acknowledgements
The authors thank Drs Farid Bekara, Florian Boissière, and Julien Gibrila for the assistance they provide in the management of the patients of this study.
Author information
Authors and Affiliations
Contributions
FB drafted the article, collected the data and supported the analysis. MB and AL provided useful comments on the article. GC co-ordinated the project, completed the analysi, provided background information and valided the final article.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval retrospective studies
The study had local review board approval from the Montpellier University Hospital research ethics committee.
Additional information
Communicated by Piet Leroy
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Blanc, F., Bigorre, M., Lamouroux, A. et al. Early needle aspiration of large infant cephalohematoma: a safe procedure to avoid esthetic complications. Eur J Pediatr 179, 265–269 (2020). https://doi.org/10.1007/s00431-019-03487-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-019-03487-5