Abstract
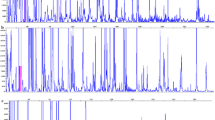
The stability, both genetic and phenotypic, of potato (Solanum tuberosum L.) cultivar Desiree plants derived from alternative propagation methodologies has been compared. Plants obtained through three clonal propagation routes—axillary-bud-proliferation, microtuberisation and a novel somatic embryogenesis system, and through true potato seeds (TPS) produced by selfing were evaluated at three levels: gross phenotype and minituber yield, changes in ploidy (measured by flow cytometry) and by molecular marker analysis [measured using AFLP (amplified fragment length polymorphism)]. The clonally propagated plants exhibited no phenotypic variation while the TPS-derived plants showed obvious phenotypic segregation. Significant differences were observed with respect to minituber yield while average plant height, at the time of harvesting, was not significantly different among plants propagated through four different routes. None of the plant types varied with respect to gross genome constitution as assessed by flow cytometry. However, a very low level of AFLP marker profile variation was seen amongst the somatic embryo (3 out of 451 bands) and microtuber (2 out of 451 bands) derived plants. Intriguingly, only AFLP markers generated using methylation sensitive restriction enzymes were found to show polymorphism. No polymorphism was observed in plants regenerated through axillary-bud-proliferation. The low level of molecular variation observed could be significant on a genome-wide scale, and is discussed in the context of possible methylation changes occurring during the process of somatic embryogenesis.





Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- ABP:
-
Axillary bud shoot plants
- AFLP:
-
Amplified fragment length polymorphism
- DAPI:
-
4′-6-Diamidino-2-phenylindole
- EMB:
-
Embling(s)
- ISSR:
-
Inter simple sequence repeat
- MS:
-
Murashige and Skoog (1962)
- MTP:
-
Microtuber plants
- NAA:
-
Naphthalene acetic acid
- TPS:
-
True potato seedling(s)
- RAPD:
-
Random amplified polymorphic DNA
- RFLP:
-
Restriction fragment length polymorphism
References
Arumuganathan K, Earle ED (1991) Estimation of nuclear DNA content of plants by flow cytometry. Plant Mol Biol Rep 9:229–233
Brar DS, Jain SM (1998) Somaclonal variation: mechanisms and applications in crop improvement. In: Jain SM, Brar DS, Ahloowalia BS (eds) Somaclonal variation and induced mutations in crop improvement. Kluwer, Boston, pp 17–37
Bryan GJ, McLean K, Bradshaw JE, Phillips M, Castelli L, De Jong WS, Waugh R (2002) Mapping QTL for resistance to the cyst nematode Globodera pallida derived from the wild potato species Solanum vernei. Theor Appl Genet 105:68–77
Cassells AC, Curry RF (2001) Oxidative stress and physiological, epigenetic and genetic variability in plant tissue culture: implications for micropropagators and genetic engineers. Plant Cell Tiss Org 64:145–157
Cecchini E, Natali L, Cavallini A, Durante M (1992) DNA variations in regenerated plants of pea (Pisum sativum L.). Theor Appl Genet 84:874–879
de Garcia E, Martinez S (1995) Somatic embryogenesis in Solanum tuberosum L. cv. Desiree from stem nodal sections. J Plant Physiol 145:526–530
Devarumath RM, Nandy S, Rani V, Marimuthu S, Muraleedharan N, Raina SN (2002) RAPD, ISSR and RFLP fingerprints as useful markers to evaluate genetic integrity of micropropagated plants of three diploid and triploid elite tea clones representing Camellia sinensis (China type) and C. assamica ssp. assamica (Assam-India type). Plant Cell Rep 21:166–173
Fiegert AK, Mix-Wagner G, Vorlop KD (2000) Regeneration of Solanum tuberosum L. cv. Tomensa: induction of somatic embryogenesis in liquid culture for the production of “artificial seed”. Landbauforsch Volk 50:199–202
Hale AL, Miller JC (2005) Suitability of AFLP and microsatellite marker analysis for discriminating intraclonal variants of the potato cultivar Russet Norkotah. J Am Soc Hortic Sci 130:624–630
Henry RJ, Nato A, de Buyser J (1998) Genetic fidelity of plants regenerated from somatic embryos of cereals. In: Jain SM, Brar DS, Ahloowalia BS (eds) Somaclonal variation and induced mutations in crop improvement. Kluwer, Boston, pp 17–37
JayaSree T, Pavan U, Ramesh M, Rao AV, Reddy KJM, Sadanandam A (2001) Somatic embryogenesis from leaf cultures of potato. Plant Cell Tiss Org 64:13–17
Karp A (1989) Can genetic instability be controlled in plant tissue cultures? Int Assoc Plant Tiss Cult Newsl 58:2–11
Karp A (1991) On the current understanding of somaclonal variation. In: Miflin BJ (ed) Oxford surveys of plant molecular and cell biology. Oxford University Press, Oxford, pp 1–58
Karp A (1995) Somaclonal variation as a tool for crop improvement. Euphytica 85:295–302
Larkin PJ, Scowcroft WR (1981) Somaclonal variation—a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60:197–214
Lo Schiavo F, Pitto L, Giuliano G, Torti G, Nutironchi V, Marazziti D, Vergara R, Orselli S, Terzi M (1989) DNA methylation of embryogenic carrot cell cultures and its variations as caused by mutation, differentiation, hormones and hypomethylating drugs. Theor Appl Genet 77:325–331
Mamiya K, Sakamoto Y, Ohnishi N, Hirosawa T (2001) Synthetic seeds of Asparagus officinalis L. In: Bhojwani SS, Soh W-Y (eds) Current trends in the embryology of angiosperms. Kluwer, Dordrecht, pp 337–352
Martins M, Sarmento D, Oliveira MM (2004) Genetic stability of micropropagated almond plantlets, as assessed by RAPD and ISSR markers. Plant Cell Rep 23:492–496
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Naik PS, Chakrabarti SK, Sarkar D, Birhman RK (2000) Potato biotechnology: Indian perspective. In: Khurana SMP, Shekhawat GS, Singh BP, Pandey SK (eds) Proceedings of Global Conference on Potato, New Delhi, December 6–11, 1999: potato, global research and development. Indian Potato Research Association, Central Potato Research Institute, Shimla, pp 194–211
Ochatt SJ, Marconi PL, Radice S, Arnozis PA, Caso OH (1998) In vitro recurrent selection of potato: production and characterization of salt tolerant cell lines and plants. Plant Cell Tiss Org 55:1–8
Odake Y, Udagawa A, Saga H, Mii M (1993) Somatic embryogenesis of tetraploid plants from internodal segments of a diploid cultivar of Asparagus officinalis L. grown in liquid culture. Plant Sci 94:173–177
Payne RW, Lane PW, Digby PGN, Harding SA, Leech PK, Morgan GW, Todd AD, Thompson R, Tunnicliffe Wilson G, Welham SJ, White RP (1993) Genstat 5 Release 3: reference manual. Oxford University Press, Oxford
Phillips RL, Kaeppler SM, Olhoft P (1994) Genetic instability of plant tissue cultures—breakdown of normal controls. Proc Natl Acad Sci USA 91:5222–5226
Ronchi VN, Giorgetti L, Tonelli M, Martini G (1992) Ploidy reduction and genome segregation in cultured carrot cell lines. 1. Prophase chromosome reduction. Plant Cell Tiss Org 30:107–114
Rugh CL, Parrott WA, Merkle SA (1993) Ploidy variation in embryogenic yellow poplar. In: Proceedings of 22nd Southern Forest Tree Improvement Conference, pp 493
Sala F, Arencibia A, Castiglione S, Christou P, Zheng Y, Han Y (1999) Molecular and field analysis of somaclonal variation in transgenic plants. In: Altman A, Ziv M, Izhar S (eds) Plant biotechnology and in vitro biology in the 21st century. Kluwer, Dordrecht, pp 259–262
Sanchez-Teyer LF, Quiroz-Figueroa F, Loyola-Vargas V, Infante D (2003) Culture induced variation in plants of Coffea arabica cv. Caturra rojo, regenerated by direct and indirect somatic embryogenesis. Mol Biotechnol 23:107–115
Schafer-Menuhr A, Mix-Wagner G, Vorlop KD (2003) Regeneration of plants from cell suspension cultures and encapsulated cell suspension cultures of Solanum tuberosum L. cv. Clarissa. Landbauforsch Volk 53:53–59
Seabrook JEA, Douglass LK (2001) Somatic embryogenesis on various potato tissues from a range of genotypes and ploidy levels. Plant Cell Rep 20:175–182
Sharma SK (2006) The development of an efficient somatic embryogenesis system for the production of synthetic seed in potato. University of Dundee, Dundee
Sharma SK, Millam S (2004) Somatic embryogenesis in Solanum tuberosum L.: a histological examination of key developmental stages. Plant Cell Rep 23:115–119
Shenoy VB, Vasil IK (1992) Biochemical and molecular analysis of plants derived from embryogenic tissue cultures of napier grass (Pennisetum purpureum K. Schum). Theor Appl Genet 83:947–955
Shoyama Y, Matsushita H, Zhu XX, Kishira H (1995) Somatic embryogenesis in ginseng (Panax species). In: Bajaj YPS (ed) Biotechnology in agriculture and forestry. Springer, Berlin, pp 344–356
Smulders MJM, Rus-Kortekaas W, Vosman B (1995) Tissue culture induced DNA methylation polymorphisms in repetitive DNA of tomato calli and regenerated plants. Theor Appl Genet 91:1257–1264
Struik PC, Wiersema SG (1999) Seed potato technology. Wageningen Pers, Wageningen
Tal M (1996) Somaclonal variation for salt tolerance in tomato and potato. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry. Springer, Berlin, pp 132–145
Vargas TE, De Garcia E, Oropeza M (2005) Somatic embryogenesis in Solanum tuberosum from cell suspension cultures: histological analysis and extracellular protein patterns. J Plant Physiol 162:449–456
Vazquez AM (2001) Insight into somaclonal variation. Plant Biosyst 135:57–62
Vos P, Hogers R, Bleeker M, Reijans M, Vandelee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP—a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Zoriniants SE, Nosov AV, Monforte-Gonzalez M, Mendes-Zeel M, Loyola-Vargas VM (2003) Variation of nuclear DNA content during somatic embryogenesis and plant regeneration of Coffea arabica L. using cytophotometry. Plant Sci 164:141–146
Acknowledgments
SKS is grateful to the Government of India and the Commonwealth Scholarship Commission, United Kingdom for his doctoral Commonwealth Scholarship Award. The authors thank Geoff Swan (SCRI) for providing the potato cv. Desiree true potato (selfed) seeds. The authors are grateful to Drs Brian Forster and Gavin Ramsay (SCRI) for critical reading of the manuscript. SCRI is supported by grant-in-aid from the Scottish Executive Environment & Rural Affairs Department (SEERAD).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, S.K., Bryan, G.J., Winfield, M.O. et al. Stability of potato (Solanum tuberosum L.) plants regenerated via somatic embryos, axillary bud proliferated shoots, microtubers and true potato seeds: a comparative phenotypic, cytogenetic and molecular assessment. Planta 226, 1449–1458 (2007). https://doi.org/10.1007/s00425-007-0583-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-007-0583-2