Abstract
Purpose
The p62, also called sequestosome 1 (SQSTM1), plays a crucial role in tumor necrosis factor (TNF)-induced optic nerve degeneration. Brimonidine has been shown to have protective effects on retinal ganglion cell bodies, although its role in their axons remains to be examined. We determined whether brimonidine modulates axonal loss induced by TNF and affects the expression of p62 in the optic nerve.
Methods
Experiments were performed on adult male Wistar rats that received an intravitreal injection of 10 ng TNF alone or simultaneous injection of TNF and 2, 20, or 200 pmol of brimonidine tartrate. The expression of p62 in the optic nerve was examined by immunoblot analysis. The effects of brimonidine on axons were evaluated by counting axon numbers 2 weeks after intravitreal injection.
Results
Intravitreal injection of brimonidine exerted substantial axonal protection against TNF-induced optic nerve degeneration. Immunoblot analysis showed that p62 was upregulated in the optic nerve after intravitreal injection of TNF, and that this increase was completely inhibited by brimonidine. Treatment with brimonidine alone also significantly decreased p62 protein levels in the optic nerve compared with the basal level.
Conclusions
These results suggest that the modulation of p62 levels in the optic nerve by brimonidine may be involved partly in its axonal protection.
Similar content being viewed by others
Introduction
Brimonidine is a α2-adrenoreceptor agonist that lowers the intraocular pressure (IOP) and is widely used for glaucoma treatment. Several studies demonstrated that brimonidine has a neuroprotective effect on retinal ganglion cells (RGCs). For example, brimonidine protected RGCs in an in vivo transgenic model of excessive oxidative stress [1] and protected RGCs against ischemia [2, 3]. It was shown that intravitreal injection of brimonidine upregulated brain-derived neurotrophic factor (BDNF) expression in the rat retina [4]. Other reports also demonstrated that brimonidine increased BDNF and p-AKT expression and protected RGCs in the ocular hypertensive rat retina [5]. Moreover, brimonidine inhibited the increases in the expression of mitochondrial transcription factor A and oxidative phosphorylation complex in the ischemic retina [6]. Those studies focused on RGC body protection, and the role brimonidine plays in their axons remains to be examined.
The p62, also called sequestosome 1 (SQSTM1), plays crucial roles in the autophagy machinery, and its accumulation has been linked to neurodegenerative disease [7–9]. Upregulated p62 was found in the compressed spinal cord, and the forced expression of p62 decreased the number of neuronal cells under hypoxic stress [10]. It was demonstrated that the overexpression of p62 promotes apoptosis with the activation of caspase-8, while knockdown of p62 reduces human glioma cell death [11]. We previously found that there was a substantial increase in p62 protein levels in optic nerve samples 1 week after IOP elevation in a rat hypertensive glaucoma model [12]. More recently, we have demonstrated that there was also an increase in p62 protein levels in the optic nerve after intravitreal injection of tumor necrosis factor (TNF) and that inhibition of p62 resulted in axonal protection in the optic nerve [13]. The TNF is involved in certain types of glaucoma [14–19], and the TNF injection model may be useful in understanding the mechanism of axonal degeneration of RGCs [20]. In the present study, we attempted to determine whether brimonidine modulates axonal loss in TNF-induced optic nerve degeneration and affects the expression of p62 in the optic nerve.
Materials and methods
Animals
Experiments were performed on 50- to 55-day-old male Wistar rats; 44 rats and 22 rats were used for the immunoblot analysis and axon counting studies, respectively. All studies were conducted according to the Association for Research in Vision and Ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Ethics Committee of the Institute of Experimental Animals of St. Marianna University Graduate School of Medicine. The animals were housed in controlled conditions, with temperature at 23 ± 1 °C, humidity at 55 ± 5 %, and light from 06:00 to 18:00.
Administration of TNF
Intravitreal injection of TNF (Sigma-Aldrich, St. Louis, MO, USA) was performed as described previously [20, 21]. Briefly, rats were anesthetized with an intramuscular injection of a mixture of ketamine-xylazine (10 and 4 mg/kg, respectively). A single 2-μl injection of 10 ng of TNF in 0.01 M PBS, pH 7.40, was administered intravitreally into the right eye of an animal under a microscope to avoid lens injury. The PBS alone was administered into the contralateral left eye as a control. In the brimonidine treatment groups, 2, 20, or 200 pmol of brimonidine tartrate (Senju Pharmaceutical Co., Ltd., Osaka, JAPAN) in 0.01 M PBS was mixed with 10 ng of TNF and administered intravitreally in a simultaneous injection. A previous study used a single 5-μl intravitreal injection of brimonidine (0.85 μM to 34 μM, i.e., 4.25 pmol to 170 pmol) in rats and showed the upregulation of BDNF in RGCs [4]. Therefore, our current brimonidine dosage (2-μl intravitreal injection of 1 μM to 100 μM) is likely to be a similar concentration. The rats were euthanized 1 or 2 weeks after the intravitreal injections with an intraperitoneal overdose of sodium pentobarbital, followed by enucleation of the eyes.
Immunoblot analysis
Forty-four rats were used for immunoblot analysis as described previously [22]. Briefly, 1 or 2 weeks after intravitreal injection, optic nerves (4 mm in length starting immediately behind the globe) were collected, homogenized, and then centrifuged at 15,000 × g for 15 min at 4 °C. Two optic nerve specimens were pooled into one sample, e.g., n = 5 included ten independent optic nerve samples per group. Protein concentrations were determined using the Bio-Rad Protein Assay kit (Bio-Rad, Hercules, CA, USA). Protein samples (5 μg per lane) were subjected to SDS-PAGE on gels (Bio-Rad) and transferred to PVDF membranes (Immobilon-P, Millipore, Billerica, MA, USA). Membranes were blocked with Tris-buffered saline (TBS)-0.1 % Tween-20 containing 5 % skim milk. Membranes were first reacted with anti-p62 antibody (1:200; Medical & Biological Laboratories Co., Nagoya, Japan) or anti-β-actin antibody (1:500; Sigma-Aldrich) in TBS containing 5 % skim milk. Membranes were then sequentially exposed to peroxidase-labeled anti-rabbit IgG antibody (Cappel, Solon, OH, USA) or peroxidase-labeled anti-mouse IgG antibody (Cappel) diluted 1:5000 in Tween-20 in TBS. Western blots were visualized with an ECL detection system (Amersham ECL Prime Western Blotting Detection Reagents, GE Healthcare, Buckinghamshire, UK).
Axon counting in optic nerves
Morphometric analysis of each optic nerve was performed as described previously with samples from 22 rats [20–22]. Eyes were obtained from the animals 2 weeks after intravitreal injection. Four-millimeter segments of the optic nerves were obtained starting 1 mm behind the globe. These segments of optic nerve were fixed by immersion in Karnovsky’s solution for 24 h at 4 °C, processed, and embedded in acrylic resin. Cross sections (1 μm thick) were cut beginning 1 mm from the globe and stained with a solution of 1 % paraphenylene-diamine (Sigma-Aldrich) in absolute methanol. For each section, images at the center and at each quadrant of the periphery (approximately 141.4 μm from the center) were acquired with a light microscope (BX51; Olympus, Tokyo, JAPAN) with a 100× coupled digital camera (MP5Mc/OL; Olympus) and associated QCapture Pro software (version 5.1, QImaging, Surrey, Canada). The acquired images were quantified using Aphelion image-processing software (version 3.2, ADCIS SA and AAI, Inc., Hérouville Saint Clair, France). The number of axons was determined in five distinct areas of 1446.5 μm2 each (each quadrant of the periphery in addition to the center; total area of 7232.3 μm2 per eye) from each eye. The number of axons per eye was averaged and expressed as the number per square millimeter. A minimum of five eyes per experimental condition was used for analysis.
Statistical analysis
Data are presented as mean ± SEM. Differences among groups were analyzed using one-way ANOVA, followed by the Mann–Whitney test. A probability value of less than 0.05 was considered to represent a statistically significant difference.
Results
Effects of brimonidine on TNF-induced axonal degeneration
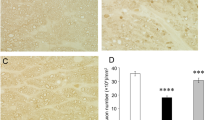
We previously demonstrated that after an approximately 30 % loss of axons 2 weeks after TNF injection, there was no further loss of axons at 1 or 2 months [20]. In the present study, compared with PBS-treated eyes (Fig. 1a), we confirmed substantial degenerative changes in the axons 2 weeks after TNF injection (Fig. 1b), which were consistent with the findings of our previous studies [20–22]. Brimonidine 20 pmol-treated eyes showed apparent attenuated effects with better-preserved nerve fibers (Fig. 1c). Substantial protective effects on axons against TNF-induced optic nerve degeneration were also seen in brimonidine 200 pmol-treated eyes (Fig. 1d). Quantitative analysis showed that treatment with brimonidine 2 pmol tended to be modestly protective, but this was not statistically significant (n = 4; p = 0.1859 versus TNF injection; Fig. 1e). Treatment with brimonidine 20 pmol exerted a significant protective effect against TNF-induced axonal loss (n = 4; p = 0.00815 versus TNF injection; Fig. 1e). Brimonidine 200 pmol-treated eyes showed 86.4 % axonal protection compared with eyes after TNF injection alone (n = 7; p = 0.00601 versus TNF injection; Fig. 1e).
Brimonidine prevented TNF-induced axon loss. Light microscopic findings 2 weeks after (a) PBS injection, (b) 10-ng TNF injection, (c) 10-ng TNF + 20-nmol brimonidine injection, or (d) 10-ng TNF +200-nmol brimonidine injection. Scale bar = 10 μm (a–d). (e) Effect of brimonidine (2–200 nmol) on axon numbers in the optic nerve. Each column represents mean ± SEM; n = 4–7 per group. *p < 0.05; **p < 0.005
Effects of brimonidine on p62 protein levels in optic nerves
We previously found that p62 was increased in optic nerve samples 1 week after TNF injection [13] at the time before axon loss became obvious. In the current study, we examined the effect of brimonidine on p62 protein levels 1 and 2 weeks after intravitreal injection, and the latter time point is when axon loss becomes obvious [20]. Consistent with our previous results [13], there was a significant increase in p62 protein levels in the optic nerve samples 1 week after TNF injection (Fig. 2a–c). This increase was completely abolished by brimonidine (Fig. 2a–c). Moreover, treatment with brimonidine alone significantly decreased p62 protein levels in the optic nerve compared with the basal level (Fig. 2d). Furthermore, there was a tendency for p62 protein levels to increase in the optic nerve samples 2 weeks after TNF injection, but this was not statistically significant (n = 4; p = 0.083265 versus PBS injection; Fig. 2e). However, this increase was completely abolished by brimonidine (Fig. 2e).
p62 protein levels in optic nerves. Immunoblot data are normalized to β-actin levels in the same sample. a Immunoblotting for p62 1 week after PBS injection, 10-ng TNF injection, or 10-ng TNF + 200-nmol brimonidine injection. b Immunoblotting for β-actin in the same sample. c Data are expressed as percentage of control. Each column represents mean ± SEM. n = 5 (10 optic nerves) per group. *p < 0.05. d Immunoblotting for p62 1 week after PBS injection or 200-nmol brimonidine injection. Data are expressed as percentage of control. Each column represents mean ± SEM. n = eight (8 optic nerves) per group. *p < 0.05. e Immunoblotting for p62 2 weeks after PBS injection, 10-ng TNF injection, or 10-ng TNF + 200-nmol brimonidine injection. Data are expressed as percentage of control. Each column represents mean ± SEM. n = eight (8 optic nerves) per group. *p < 0.05
Discussion
In the present study, brimonidine exerted a substantial protective effect against axonal loss after TNF injection. Axonal protection by brimonidine was also seen in different optic nerve injury models. For example, pretreatment with brimonidine significantly reduced axonal loss in an ischemic optic neuropathy model [23]. This is consistent with the results of a previous study showing that systemic brimonidine, which did not affect IOP, exerted substantial axonal protection following ocular hypertension [24]. That study found that systemic brimonidine ameliorated anterograde transport deficits due to ocular hypertension [24]. Thus, it is possible that the protective effects of brimonidine are associated with its improvement of anterograde and retrograde transport [25, 26]. In addition to axonal protection, axonal regeneration by brimonidine was reported. It was shown that treatment with brimonidine resulted in enhanced axonal growth in juvenile, glaucomatous, and optic nerve crush retinas in vitro [27]. A recent in vivo study has determined that Erk1/2 activity is required for brimonidine-mediated axonal regeneration after optic nerve injury [28]. Thus, it is likely that brimonidine has beneficial effects on axonal protection as well as axonal regeneration in several distinct types of optic nerve damage.
A constitutively high level of p62 under pathological conditions leads to the accumulation of damaged mitochondria and subsequent reactive oxygen species production [29], and the accumulation of p62 after autophagy inhibition causes a delay in the clearance of short-lived ubiquitin-proteasome system-specific substrates, like p53, which may mediate toxicity [30]. In the present study, we observed an increase in the p62 protein level in the optic nerve after TNF injection, consistent with the results of our previous study [13], and this increase was completely inhibited by the simultaneous injection of brimonidine. It is interesting to note that autophagosome formation was increased in response to β2-agonist administration in rat skeletal muscle [31]. Augmented formation of autophagosomes was also observed in the K562 cell line following α1-agonist treatment [32]. Therefore, it is reasonable to speculate that adrenoceptor stimulation may affect autophagy machinery, although further studies will be needed to clarify the involvement of α-adrenergic receptor regulation within the optic nerve. Because p62 accumulates when autophagy is inhibited, and decreased levels can be observed when autophagy is induced, p62 may be used as a marker of autophagy flux [33]. It was shown that β-adrenoceptor stimulation enhanced autophagic flux by promoting lysosomal degradation in fat cells [34]. It was also reported that norepinephrine strongly enhances autophagic flux in cultured cardiac fibroblasts [35]. The level of p62 may be dependent on the balance between the incoming flux, which is the transcriptional regulation in response to various stimuli, and outgoing flux, which is influenced by autophagic activity [36]. Since we found that brimonidine decreased p62 levels in the optic nerve compared with the basal level, one hypothesis posits that this may be because brimonidine directly affects p62 expression, such as by increasing autophagic flux, rather than indirectly exerting effects resulting from axonal protection. Taking the various results together, it is possible that brimonidine exerts axonal protection with the involvement of autophagy machinery.
In conclusion, the present study showed that the modulation of p62 levels by brimonidine in the optic nerve might be involved in part in its axonal protective effects. Further studies are needed to clarify the mechanism by which brimonidine alters p62 expression.
References
Levkovitch-Verbin H, Harris-Cerruti C, Groner Y, Wheeler LA, Schwartz M, Yoles E (2000) RGC death in mice after optic nerve crush injury: oxidative stress and neuroprotection. Invest Ophthalmol Vis Sci 41:4169–4174
Lafuente MP, Villegas-Pérez MP, Sobrado-Calvo P, García-Avilés A, Miralles de Imperial J, Vidal-Sanz M (2001) Neuroprotective effects of alpha(2)-selective adrenergic agonists against ischemia-induced retinal ganglion cell death. Invest Ophthalmol Vis Sci 42:2074–2084
Lafuente MP, Villegas-Pérez MP, Mayor S, Aguilera ME, Miralles de Imperial J, Vidal-Sanz M (2002) Neuroprotective effects of brimonidine against transient ischemia-induced retinal ganglion cell death: a dose response in vivo study. Exp Eye Res 74:181–189
Gao H, Qiao X, Cantor LB, WuDunn D (2002) Up-regulation of brain-derived neurotrophic factor expression by brimonidine in rat retinal ganglion cells. Arch Ophthalmol 120:797–803
Kim HS, Chang YI, Kim JH, Park CK (2007) Alteration of retinal intrinsic survival signal and effect of alpha2-adrenergic receptor agonist in the retina of the chronic ocular hypertension rat. Vis Neurosci 24:127–139
Lee D, Kim KY, Noh YH, Chai S, Lindsey JD, Ellisman MH, Weinreb RN, Ju WK (2012) Brimonidine blocks glutamate excitotoxicity-induced oxidative stress and preserves mitochondrial transcription factor A in ischemic retinal injury. PLoS One 7:e47098
Zatloukal K, Stumptner C, Fuchsbichler A, Heid H, Schnoelzer M, Kenner L, Kleinert R, Prinz M, Aguzzi A, Denk H (2002) p62 is a common component of cytoplasmic inclusions in protein aggregation diseases. Am J Pathol 160:255–263
Kuusisto E, Salminen A, Alafuzoff I (2002) Early accumulation of p62 in neurofibrillary tangles in Alzheimer’s disease: possible role in tangle formation. Neuropathol Appl Neurobiol 28:228–237
Gal J, Ström AL, Kilty R, Zhang F, Zhu H (2007) p62 accumulates and enhances aggregate formation in model systems of familial amyotrophic lateral sclerosis. J Biol Chem 282:11068–11077
Tanabe F, Yone K, Kawabata N, Sakakima H, Matsuda F, Ishidou Y, Maeda S, Abematsu M, Komiya S, Setoguchi T (2011) Accumulation of p62 in degenerated spinal cord under chronic mechanical compression: functional analysis of p62 and autophagy in hypoxic neuronal cells. Autophagy 7:1462–1471
Zhang YB, Gong JL, Xing TY, Zheng SP, Ding W (2013) Autophagy protein p62/SQSTM1 is involved in HAMLET-induced cell death by modulating apoptosis in U87MG cells. Cell Death Dis 4:e550
Kitaoka Y, Munemasa Y, Kojima K, Hirano A, Ueno S, Takagi H (2013) Axonal protection by Nmnat3 overexpression with involvement of autophagy in optic nerve degeneration. Cell Death Dis 4:e860
Kojima K, Kitaoka Y, Munemasa Y, Hirano A, Sase K, Takagi H (2014) Axonal protection by modulation of p62 expression in TNF-induced optic nerve degeneration. Neurosci Lett 581:37–41
Yan X, Tezel G, Wax MB, Edward DP (2000) Matrix metalloproteinases and tumor necrosis factor alpha in glaucomatous optic nerve head. Arch Ophthalmol 118:666–678
Yuan L, Neufeld AH (2000) Tumor necrosis factor-alpha: a potentially neurodestructive cytokine produced by glia in the human glaucomatous optic nerve head. Glia 32:42–50
Tezel G, Wax MB (2000) Increased production of tumor necrosis factor-α by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induced apoptosis in cocultured retinal ganglion cells. J Neurosci 20:8693–8700
Tezel G, Li LY, Patil RV, Wax MB (2001) TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Invest Ophthalmol Vis Sci 42:1787–1794
Nakazawa T, Nakazawa C, Matsubara A, Noda K, Hisatomi T, She H, Michaud N, Hafezi-Moghadam A, Miller JW, Benowitz LI (2006) Tumor necrosis factor-α mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci 26:12633–12641
Sawada H, Fukuchi T, Tanaka T, Abe H (2010) Tumor necrosis factor-α concentrations in the aqueous humor of patients with glaucoma. Invest Ophthalmol Vis Sci 51:903–906
Kitaoka Y, Kitaoka Y, Kwong JMK, Ross-Cisneros FN, Wang J, Tsai RK, Sadun AA, Lam TT (2006) TNF-α-induced optic nerve degeneration and nuclear factor-κB p65. Invest Ophthalmol Vis Sci 47:1448–1457
Kitaoka Y, Hayashi Y, Kumai T, Takeda H, Munemasa Y, Fujino H, Kitaoka Y, Ueno S, Sadun AA, Lam TT (2009) Axonal and cell body protection by nicotinamide adenine dinucleotide in tumor necrosis factor-induced optic neuropathy. J Neuropathol Exp Neurol 68:915–927
Kitaoka Y, Munemasa Y, Hayashi Y, Kuribayashi J, Koseki N, Kojima K, Kumai T, Ueno S (2011) Axonal protection by 17β-estradiol through thioredoxin-1 in tumor necrosis factor-induced optic neuropathy. Endocrinology 152:2775–2785
Danylkova NO, Alcala SR, Pomeranz HD, McLoon LK (2007) Neuroprotective effects of brimonidine treatment in a rodent model of ischemic optic neuropathy. Exp Eye Res 84:293–301
Lambert WS, Ruiz L, Crish SD, Wheeler LA, Calkins DJ (2011) Brimonidine prevents axonal and somatic degeneration of retinal ganglion cell neurons. Mol Neurodegener 6:4
Avilés-Trigueros M, Mayor-Torroglosa S, García-Avilés A, Lafuente MP, Rodríguez ME, Miralles de Imperial J, Villegas-Pérez MP, Vidal-Sanz M (2003) Transient ischemia of the retina results in massive degeneration of the retinotectal projection: long-term neuroprotection with brimonidine. Exp Neurol 184:767–777
Lafuente López-Herrera MP, Mayor-Torroglosa S, Miralles de Imperial J, Villegas-Pérez MP, Vidal-Sanz M (2002) Transient ischemia of the retina results in altered retrograde axoplasmic transport: neuroprotection with brimonidine. Exp Neurol 178:243–258
Prokosch V, Panagis L, Volk GF, Dermon C, Thanos S (2010) Alpha2-adrenergic receptors and their core involvement in the process of axonal growth in retinal explants. Invest Ophthalmol Vis Sci 51:6688–6699
Fujita Y, Sato A, Yamashita T (2013) Brimonidine promotes axon growth after optic nerve injury through Erk phosphorylation. Cell Death Dis 4:e763
Johansen T, Lamark T (2011) Selective autophagy mediated by autophagic adapter proteins. Autophagy 7:279–296
Korolchuk VI, Mansilla A, Menzies FM, Rubinsztein DC (2009) Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Mol Cell 33:517–527
Joassard OR, Amirouche A, Gallot YS, Desgeorges MM, Castells J, Durieux AC, Berthon P, Freyssenet DG (2013) Regulation of Akt-mTOR, ubiquitin-proteasome and autophagy-lysosome pathways in response to formoterol administration in rat skeletal muscle. Int J Biochem Cell Biol 45:2444–2455
Fuchs R, Schraml E, Leitinger G, Stelzer I, Allard N, Haas HS, Schauenstein K, Sadjak A (2011) α1-Adrenergic drugs modulate differentiation and cell death of human erythroleukemia cells through non adrenergic mechanism. Exp Cell Res 317:2239–2251
Bjørkøy G, Lamark T, Pankiv S, Øvervatn A, Brech A, Johansen T (2009) Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol 452:181–197
Lizaso A, Tan KT, Lee YH (2013) β-adrenergic receptor-stimulated lipolysis requires the RAB7-mediated autolysosomal lipid degradation. Autophagy 9:1228–1243
Aránguiz-Urroz P, Canales J, Copaja M, Troncoso R, Vicencio JM, Carrillo C, Lara H, Lavandero S, Díaz-Araya G (2011) Beta(2)-adrenergic receptor regulates cardiac fibroblast autophagy and collagen degradation. Biochim Biophys Acta 1812:23–31
Puissant A, Fenouille N, Auberger P (2012) When autophagy meets cancer through p62/SQSTM1. Am J Cancer Res 2:397–413
Acknowledgments
This work was supported by Grants-in-Aid No. 24592683 (YK) and No. 23792016 (YM) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. We thank Ms. Yukari Hara for skillful technical support.
Disclosure statement
The authors have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kitaoka, Y., Kojima, K., Munemasa, Y. et al. Axonal protection by brimonidine with modulation of p62 expression in TNF-induced optic nerve degeneration. Graefes Arch Clin Exp Ophthalmol 253, 1291–1296 (2015). https://doi.org/10.1007/s00417-015-3005-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-3005-3