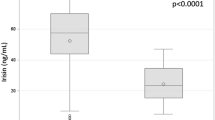
Abstract
Objective
To assess the changes of muscle-related biomarkers at the early stage of amyotrophic lateral sclerosis, and to confirm these findings in an experimental animal model.
Methods
Thirty-nine subjects with sporadic amyotrophic lateral sclerosis and 20 healthy controls were enrolled and longitudinally evaluated. We evaluated serum creatine kinase and creatinine levels and appendicular lean soft-tissue mass using dual X-ray absorptiometry. The levels of biomarkers at early ALS stages were estimated using linear mixed models with unstructured correlation and random intercepts. We also analyzed the longitudinal changes of serum creatine kinase and creatinine, together with the mRNA levels of acetylcholine receptor subunit γ (Chrng) and muscle-associated receptor tyrosine kinase, markers of denervation, in the gastrocnemius muscle of superoxide dismutase 1 (SOD1)G93A transgenic mice, an animal model of amyotrophic lateral sclerosis.
Results
The estimated levels of creatine kinase were higher in subjects with amyotrophic lateral sclerosis at the early stage than in healthy controls, although the estimated appendicular lean soft-tissue mass and creatinine levels were equivalent between both groups, suggesting that the elevation of creatine kinase precedes both muscular atrophy and subjective motor symptoms in sporadic amyotrophic lateral sclerosis. In SOD1G93A mice, the serum levels of creatine kinase were elevated at 9 weeks of age (peri-onset) when Chrng started to be up-regulated, and were then down-regulated at 15 weeks of age, consistent with the clinical data from patients with sporadic amyotrophic lateral sclerosis.
Interpretation
Creatine kinase elevation precedes muscular atrophy and reflects muscle denervation at the early stage.





Similar content being viewed by others
References
Rowland L, Shneider N (2001) Amyotrophic lateral sclerosis. N Engl J Med 344:1688–1700
Wijesekera L, Leigh P (2009) Amyotrophic lateral sclerosis. Orphanet J Rare Dis 4:3
Renton AE, Chiò A, Traynor BJ (2014) State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci 17:17–23
Nakamura R, Sone J, Atsuta N et al (2016) Next-generation sequencing of 28 ALS-related genes in a Japanese ALS cohort. Neurobiol Aging 39:219.e1–219.e8
Taylor JP, Brown RH, Cleveland DW (2016) Decoding ALS: from genes to mechanism. Nature 539:197–206
Bensimon G, Lacomblez L, Meininger V (1994) A controlled trial of riluzole in amyotrophic lateral sclerosis. N Engl J Med 330:585–591
Abe K, Itoyama Y, Sobue G et al (2014) Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Front Degener 15:610–617
Villemagne VL, Burnham S, Bourgeat P et al (2013) Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol 12:357–367
Cooper CA, Chahine LM (2016) Biomarkers in prodromal parkinson disease: a qualitative review. J Int Neuropsychol Soc 22:956–967
Vos SJB, Xiong C, Visser PJ et al (2013) Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol 12:957–965
Katsuno M, Tanaka F, Sobue G (2012) Perspectives on molecular targeted therapies and clinical trials for neurodegenerative diseases. J Neurol Neurosurg Psychiatry 83:329–335
Hijikata Y, Hashizume A, Yamada S et al (2018) Biomarker-based analysis of preclinical progression in spinal and bulbar muscular atrophy. Neurology. https://doi.org/10.1212/WNL.0000000000005360
Solomon A, Mangialasche F, Richard E et al (2014) Advances in the prevention of Alzheimer’s disease and dementia. J Intern Med 275:229–250
Schapira AHV, Olanow CW, Greenamyre JT, Bezard E (2014) Slowing of neurodegeneration in Parkinson’s disease and Huntington’s disease: Future therapeutic perspectives. Lancet 384:545–555
Lehmer C, Oeckl P, Weishaupt JH et al (2017) Poly-GP in cerebrospinal fluid links C9orf72 -associated dipeptide repeat expression to the asymptomatic phase of ALS/FTD. EMBO Mol Med 9:859–868
Freischmidt A, Müller K, Zondler L et al (2014) Serum microRNAs in patients with genetic amyotrophic lateral sclerosis and pre-manifest mutation carriers. Brain 137:2938–2950
Vucic S, Nicholson GA, Kiernan MC (2008) Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain 131:1540–1550
Aggarwal A, Nicholson G (2002) Detection of preclinical motor neurone loss in SOD1 mutation carriers using motor unit number estimation. J Neurol Neurosurg Psychiatry 73:199–201
Gallo V, Wark PA, Jenab M et al (2013) Prediagnostic body fat and risk of death from amyotrophic lateral sclerosis: the EPIC cohort. Neurology 80:829–838
Mariosa D, Hammar N, Malmström H et al (2017) Blood biomarkers of carbohydrate, lipid, and apolipoprotein metabolisms and risk of amyotrophic lateral sclerosis: a more than 20-year follow-up of the Swedish AMORIS cohort. Ann Neurol 81:718–728
Ohashi Y, Tashiro K, Itoyama Y et al (2001) Study of functional rating scale for amyotrophic lateral sclerosis: revised ALSFRS(ALSFRS-R) Japanese version. No To Shinkei 53:346–355
Hashizume A, Katsuno M, Suzuki K et al (2015) A functional scale for spinal and bulbar muscular atrophy: cross-sectional and longitudinal study. Neuromuscul Disord 25:554–562
Haarbo J, Gotfredsen A, Hassager C, Christiansen C (1991) Validation of body composition by dual energy X-ray absorptiometry (DEXA). Clin Physiol 11:331–341
Hijikata Y, Katsuno M, Suzuki K et al (2016) Impaired muscle uptake of creatine in spinal and bulbar muscular atrophy. Ann Clin Transl Neurol 3:537–546
Gurney ME, Pu H, Chiu AY et al (1994) Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264:1772–1775
Leitner M, Menzies S, Lutz C (2009) Working with ALS mice. Jax. http://www.alsresearchforum.org/wp-content/uploads/p4l_jax_sod1manual_20091202_29aPcx-1.pdf. Accessed 24 Aug 2019
Rocchi A, Milioto C, Parodi S et al (2016) Glycolytic-to-oxidative fiber-type switch and mTOR signaling activation are early-onset features of SBMA muscle modified by high-fat diet. Acta Neuropathol 132:127–144
Yau W-YW, Tudorascu DL, McDade EM et al (2015) Longitudinal assessment of neuroimaging and clinical markers in autosomal dominant Alzheimer’s disease: a prospective cohort study. Lancet Neurol 14:804–813
Chan YH (2003) Biostatistics 104: correlation analysis. Singap Med J 44:614–619
Wyss M, Kaddurah-Daouk R (2000) Creatine and creatinine metabolism. Physiol Rev 80:1107–1213
Panitch HS, Franklin GM (1972) Elevation of serum creatine phosphokinase in amyotrophic lateral sclerosis. Neurology 22:964–966
Harrington TM, Cohen MD, Bartleson JD, Ginsburg WW (1983) Elevation of creatine kinase in amyotrophic lateral sclerosis. Potential confusion with pols. Potential confusion with polmyositis. Arthritis Rheum 26:201–205
Gibson SB, Kasarskis EJ, Hu N et al (2015) Relationship of creatine kinase to body composition, disease state, and longevity in ALS. Amyotroph Lateral Scler Front Degener 16:473–477
Felice KJ, North WA (1998) Creatine kinase values in amyotrophic lateral sclerosis. J Neurol Sci 160:3–5
Iłzecka J, Stelmasiak Z (2003) Creatine kinase activity in amyotrophic lateral sclerosis patients. Neurol Sci 24:286–287
Rafiq MK, Lee E, Bradburn M et al (2016) Creatine kinase enzyme level correlates positively with serum creatinine and lean body mass, and is a prognostic factor for survival in amyotrophic lateral sclerosis. Eur J Neurol 23:1071–1078
Tai H, Cui L, Guan Y et al (2017) Correlation of creatine kinase levels with clinical features and survival in amyotrophic lateral sclerosis. Front Neurol 8:1–5
Tai H, Cui L, Liu M et al (2018) Creatine kinase level and its relationship with quantitative electromyographic characteristics in amyotrophic lateral sclerosis. Clin Neurophysiol 129:926–930
Satoh J, Okada K, Kishi T et al (2000) Cramping pain and prolonged elevation of serum creatine kinase levels in a patient with Guillain-Barré syndrome following Campylobacter jejuni enteritis. Eur J Neurol 7:107–109
Linkhart TA, Wilson BW (1975) Appearance of acetylcholinesterase and creatine kinase in plasma of normal chickens after denervation. J Neurol Sci 26:193–201
Pun S, Santos AF, Saxena S et al (2006) Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci 9:408–419
Kim J, So W-Y (2018) High body mass index is associated with the extent of muscle damage after eccentric exercise. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph15071378
Eisen A, Kiernan M, Mitsumoto H, Swash M (2014) Amyotrophic lateral sclerosis: a long preclinical period? J Neurol Neurosurg Psychiatry 85:1232–1238
Williams AH, Valdez G, Moresi V et al (2009) MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science 326:1549–1554
Bruneteau G, Simonet T, Bauché S et al (2013) Muscle histone deacetylase 4 upregulation in amyotrophic lateral sclerosis: Potential role in reinnervation ability and disease progression. Brain 136:2359–2368
Andrés V, Cussó R, Carreras J (1990) Effect of denervation on the distribution and developmental transition of phosphoglycerate mutase and creatine phosphokinase isozymes in rat muscles of different fiber-type composition. Differentiation 43:98–103
Dupuis L, Corcia P, Fergani A et al (2008) Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology 70:1004–1009
Ikeda K, Hirayama T, Takazawa T et al (2012) Relationships between disease progression and serum levels of lipid, urate, creatinine and ferritin in Japanese patients with amyotrophic lateral sclerosis: a cross-sectional study. Intern Med 51:1501–1508
Ogaki K, Li Y, Atsuta N et al (2012) Analysis of C9orf72 repeat expansion in 563 Japanese patients with amyotrophic lateral sclerosis. Neurobiol Aging 33:11–16
Acknowledgements
This work was funded by a Grant-in-Aid (KAKENHI) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 17H04195); grants from the Japan Agency for Medical Research and Development (Nos. 17ek0109221h0001 and 18ek0109221h0002); a Grant from the Naito Foundation; and a Grant from the Hori Sciences and Arts Foundation.
Author information
Authors and Affiliations
Contributions
DI, AH, and MK conceived and designed the study. DI, AH, YH, SY, YK, and HM contributed to the acquisition of clinical data. DI, MI, and YI performed animal experiments. DI and MK performed analysis and interpretation of the data. AH performed statistical analysis. DI drafted the manuscript, and AH and MK revised it for intellectual content.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors have no relevant conflicts of interest to report.
Ethical standards
This study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments, the Ethics Guidelines for Human Genome/Gene Analysis Research, and the Ethical Guidelines for Medical and Health Research Involving Human Subjects endorsed by the Japanese government. This study was approved by the Ethics Review Committee of Nagoya University Graduate School of Medicine (Nos. 2013–0035 and 2015–0041), and all participants gave written informed consent before participation. All of the animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and under the approval of the Nagoya University Animal Experiment Committee (No. 29170).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ito, D., Hashizume, A., Hijikata, Y. et al. Elevated serum creatine kinase in the early stage of sporadic amyotrophic lateral sclerosis. J Neurol 266, 2952–2961 (2019). https://doi.org/10.1007/s00415-019-09507-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09507-6