Abstract
Background
The diagnosis of atypical inflammatory demyelinating lesions can be difficult. Brain biopsy is often required to exclude neoplasms. Moreover, the relationship between these lesions and multiple sclerosis and NMOSD is not clear.
Objectives
Our objectives were to describe radiological and pathological characteristics of patients with acute inflammatory demyelinating lesions.
Methods
We retrospectively identified patients with brain biopsy performed for diagnostic uncertainty revealing a demyelinating lesion. A complete clinical, biological, radiological and pathological analysis was performed.
Results
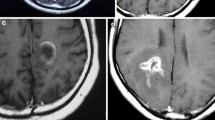
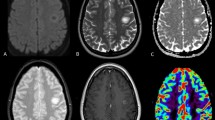
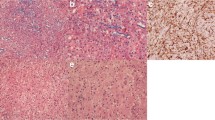
Twenty patients (15 with a single lesion) were included. MRI disclosed a wide range of lesions including infiltrative lesions (40%), ring-like lesion (15%) Baló-like lesion (15%) and acute haemorrhagic leukoencephalitis (20%). In spite of a marked heterogeneity, some findings were common: a peripheral B1000 hyperintense rim (70%), a slight oedema with mild mass effect (75%) and an open-rim peripheral enhancement (75%). Histopathology revealed that all cases featured macrophages distributed throughout, extensive demyelination, axonal preservation and absence of haemorrhagic changes. In the majority of cases, macrophages were the predominant inflammatory infiltrate and astrocytes were reactive and dystrophic. Aquaporin-4 staining was systematically preserved. After a mean follow-up of 5 years (1–12), 16/20 patients had a diagnosis of monophasic acute atypical inflammatory demyelinating lesion. One patient was diagnosed with MS and 3 with AQP4 negative NMOSD.
Discussion
Although imaging findings in patients with atypical inflammatory demyelinating lesions are heterogeneous, some common features such as peripheral DWI hyperintense rim with open-rim enhancement and absence of oedema argue in favour of a demyelinating lesion and should preclude a brain biopsy. In this context, AQP4 staining is systematically preserved and argues against an AQP4-positive NMOSD. Moreover, long-term follow-up is characterized by low recurrence rate.



Similar content being viewed by others
Abbreviations
- AHLE:
-
Acute haemorrhagic leukoencephalitis
- AQP4:
-
Aquaporin-4
- AIIDL:
-
Atypical idiopathic inflammatory demyelinating lesion
- CNS:
-
Central nervous system
- DWI:
-
Diffusion-weighted imaging
- FLAIR:
-
Fluid attenuated inversion recovery
- GFAP:
-
Glial fibrillary acid protein
- IgG:
-
Immunoglobulin G
- LFB:
-
Luxol fast blue
- MOG:
-
Myelin oligodendrocyte glycoprotein
- MS:
-
Multiple sclerosis
- NMO:
-
Neuromyelitis optica
- NMOSD:
-
Neuromyelitis optica spectrum disorders
- ON:
-
Optic neuritis
References
Hardy TA, Reddel SW, Barnett MH et al (2016) Atypical inflammatory demyelinating syndromes of the CNS. Lancet Neurol 15:967–981. https://doi.org/10.1016/S1474-4422(16)30043-6
Hardy TA, Miller DH (2014) Baló’s concentric sclerosis. Lancet Neurol 13:740–746. https://doi.org/10.1016/S1474-4422(14)70052-3
Hardy TA, Chataway J (2013) Tumefactive demyelination: an approach to diagnosis and management. J Neurol Neurosurg Psychiatry 84:1047–1053. https://doi.org/10.1136/jnnp-2012-304498
Uehara T, Beck G, Baba K et al (2016) Tumefactive brain lesion with rapid cavity formation associated with anti-aquaporin-4 antibody. Neurol Neuroimmunol Neuroinflammation 3:e230. https://doi.org/10.1212/NXI.0000000000000230
Wang JJ, Jaunmuktane Z, Mummery C et al (2016) Inflammatory demyelination without astrocyte loss in MOG antibody-positive NMOSD. Neurology 87:229–231. https://doi.org/10.1212/WNL.0000000000002844
Popescu BFG, Guo Y, Jentoft ME et al (2015) Diagnostic utility of aquaporin-4 in the analysis of active demyelinating lesions. Neurology 84:148–158. https://doi.org/10.1212/WNL.0000000000001126
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302. https://doi.org/10.1002/ana.22366
Wingerchuk DM, Banwell B, Bennett JL et al (2015) International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 85:177–189. https://doi.org/10.1212/WNL.0000000000001729
Siri A, Carra-Dalliere C, Ayrignac X et al (2015) Isolated tumefactive demyelinating lesions: diagnosis and long-term evolution of 16 patients in a multicentric study. J Neurol 262:1637–1645. https://doi.org/10.1007/s00415-015-7758-8
Lucchinetti CF, Gavrilova RH, Metz I et al (2008) Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain J Neurol 131:1759–1775. https://doi.org/10.1093/brain/awn098
Jeong IH, Kim S-H, Hyun J-W et al (2015) Tumefactive demyelinating lesions as a first clinical event: clinical, imaging, and follow-up observations. J Neurol Sci 358:118–124. https://doi.org/10.1016/j.jns.2015.08.034
Seewann A, Enzinger C, Filippi M et al (2008) MRI characteristics of atypical idiopathic inflammatory demyelinating lesions of the brain: a review of reported findings. J Neurol 255:1–10. https://doi.org/10.1007/s00415-007-0754-x
Kuhlmann T, Ludwin S, Prat A et al (2017) An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol (Berl) 133:13–24. https://doi.org/10.1007/s00401-016-1653-y
Brück W, Popescu B, Lucchinetti CF et al (2012) Neuromyelitis optica lesions may inform multiple sclerosis heterogeneity debate. Ann Neurol 72:385–394. https://doi.org/10.1002/ana.23621
Misu T, Fujihara K, Kakita A et al (2007) Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain J Neurol 130:1224–1234. https://doi.org/10.1093/brain/awm047
Roemer SF, Parisi JE, Lennon VA et al (2007) Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain J Neurol 130:1194–1205. https://doi.org/10.1093/brain/awl371
Altintas A, Petek B, Isik N et al (2012) Clinical and radiological characteristics of tumefactive demyelinating lesions: follow-up study. Mult Scler Houndmills Basingstoke Engl 18:1448–1453. https://doi.org/10.1177/1352458512438237
Koelblinger C, Fruehwald-Pallamar J, Kubin K et al (2013) Atypical idiopathic inflammatory demyelinating lesions (IIDL): conventional and diffusion-weighted MR imaging (DWI) findings in 42 cases. Eur J Radiol 82:1996–2004. https://doi.org/10.1016/j.ejrad.2013.07.026
Hiremath SB, Muraleedharan A, Kumar S et al (2017) Combining diffusion tensor metrics and DSC perfusion imaging: can it improve the diagnostic accuracy in differentiating tumefactive demyelination from high-grade glioma? AJNR Am J Neuroradiol 38:685–690. https://doi.org/10.3174/ajnr.A5089
Wallner-Blazek M, Rovira A, Fillipp M et al (2013) Atypical idiopathic inflammatory demyelinating lesions: prognostic implications and relation to multiple sclerosis. J Neurol 260:2016–2022. https://doi.org/10.1007/s00415-013-6918-y
Renard D, Castelnovo G, Campello C et al (2014) An MRI review of acquired corpus callosum lesions. J Neurol Neurosurg Psychiatry 85:1041–1048. https://doi.org/10.1136/jnnp-2013-307072
Garg N, Reddel SW, Miller DH et al (2015) The corpus callosum in the diagnosis of multiple sclerosis and other CNS demyelinating and inflammatory diseases. J Neurol Neurosurg Psychiatry 86:1374–1382. https://doi.org/10.1136/jnnp-2014-309649
Lu S-S, Kim SJ, Kim HS et al (2014) Utility of proton MR spectroscopy for differentiating typical and atypical primary central nervous system lymphomas from tumefactive demyelinating lesions. AJNR Am J Neuroradiol 35:270–277. https://doi.org/10.3174/ajnr.A3677
Toh CH, Wei K-C, Chang C-N et al (2013) Differentiation of primary central nervous system lymphomas and glioblastomas: comparisons of diagnostic performance of dynamic susceptibility contrast-enhanced perfusion MR imaging without and with contrast-leakage correction. AJNR Am J Neuroradiol 34:1145–1149. https://doi.org/10.3174/ajnr.A3383
Kim DS, Na DG, Kim KH et al (2009) Distinguishing tumefactive demyelinating lesions from glioma or central nervous system lymphoma: added value of unenhanced CT compared with conventional contrast-enhanced MR imaging. Radiology 251:467–475. https://doi.org/10.1148/radiol.2512072071
Hu W, Lucchinetti CF (2009) The pathological spectrum of CNS inflammatory demyelinating diseases. Semin Immunopathol 31:439–453. https://doi.org/10.1007/s00281-009-0178-z
Mandler RN, Davis LE, Jeffery DR, Kornfeld M (1993) Devic’s neuromyelitis optica: a clinicopathological study of 8 patients. Ann Neurol 34:162–168. https://doi.org/10.1002/ana.410340211
Takano R, Misu T, Takahashi T et al (2010) Astrocytic damage is far more severe than demyelination in NMO: a clinical CSF biomarker study. Neurology 75:208–216. https://doi.org/10.1212/WNL.0b013e3181e2414b
Morales Y, Parisi JE, Lucchinetti CF (2006) The pathology of multiple sclerosis: evidence for heterogeneity. Adv Neurol 98:27–45
Ando M, Yamashiro K, Noda K, Okuma Y (2011) Aquaporin-4 autoimmunity can cause exclusive brain tumefactive lesions. Intern Med Tokyo Jpn 50:2437–2438
Matsuoka T, Suzuki SO, Iwaki T et al (2010) Aquaporin-4 astrocytopathy in Baló’s disease. Acta Neuropathol (Berl) 120:651–660. https://doi.org/10.1007/s00401-010-0733-7
Matsuoka T, Suzuki SO, Suenaga T et al (2011) Reappraisal of aquaporin-4 astrocytopathy in Asian neuromyelitis optica and multiple sclerosis patients. Brain Pathol Zurich Switz 21:516–532. https://doi.org/10.1111/j.1750-3639.2011.00475.x
Masuda H, Mori M, Katayama K et al (2013) Anti-aquaporin-4 antibody-seronegative NMO spectrum disorder with Baló’s concentric lesions. Intern Med Tokyo Jpn 52:1517–1521
Fargeot G, Aboab J, Savatovsky J et al (2018) Central nervous system Aquaporin4 autoimmunity revealed by a single pseudotumoral encephalic lesion. Metab Brain Dis 33:353–355. https://doi.org/10.1007/s11011-017-0141-y
Kim S-H, Kim W, Kook M-C et al (2012) Central nervous system aquaporin-4 autoimmunity presenting with an isolated cerebral abnormality. Mult Scler Houndmills Basingstoke Engl 18:1340–1343. https://doi.org/10.1177/1352458512441271
Jurynczyk M, Geraldes R, Probert F et al (2017) Distinct brain imaging characteristics of autoantibody-mediated CNS conditions and multiple sclerosis. Brain J Neurol 140:617–627. https://doi.org/10.1093/brain/aww350
Author information
Authors and Affiliations
Contributions
XA: study concept and design, acquisition of data, analysis and interpretation, and writing. VR: laboratory analysis, critical revision of the manuscript for important intellectual content. BL: laboratory analysis, critical revision of the manuscript for important intellectual content. TV: laboratory analysis, critical revision of the manuscript for important intellectual content. NMdeC: acquisition of data, critical revision of the manuscript for important intellectual content. CC-D: acquisition of data, critical revision of the manuscript for important intellectual content. MC: acquisition of data, critical revision of the manuscript for important intellectual content. NC: acquisition of data, critical revision of the manuscript for important intellectual content. JdeS: acquisition of data, critical revision of the manuscript for important intellectual content. SH: acquisition of data, critical revision of the manuscript for important intellectual content. GA: acquisition of data, critical revision of the manuscript for important intellectual content. HO: acquisition of data, critical revision of the manuscript for important intellectual content. FC: acquisition of data, critical revision of the manuscript for important intellectual content. FD-D: acquisition of data, critical revision of the manuscript for important intellectual content. RM: acquisition of data, critical revision of the manuscript for important intellectual content. FT: acquisition of data, critical revision of the manuscript for important intellectual content. MC: acquisition of data, critical revision of the manuscript for important intellectual content. A-MG: acquisition of data, critical revision of the manuscript for important intellectual content. AK: acquisition of data, critical revision of the manuscript for important intellectual content. GE: acquisition of data, critical revision of the manuscript for important intellectual content. BC-N, TA: acquisition of data, critical revision of the manuscript for important intellectual content. DS: acquisition of data, critical revision of the manuscript for important intellectual content. ET: acquisition of data, critical revision of the manuscript for important intellectual content. AR: acquisition of data, critical revision of the manuscript for important intellectual content. LM: acquisition of data, critical revision of the manuscript for important intellectual content. M-P, B-M: acquisition of data, critical revision of the manuscript for important intellectual content. PL: study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content. SK: study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Conflicts of interest
Xavier Ayrignac has received consulting and lecturing fees, travel grants and research support from Bayer-Schering, Biogen Idec, Genzyme, Novartis, Merck Serono, Roche, and Teva Pharma. Valérie Rigau reports no disclosure relevant to this manuscript. Benoit Lhermitte reports no disclosure relevant to this manuscript. Thierry Vincent reports no disclosure relevant to this manuscript. Nicolas Menjot de Champfleur reports no disclosure relevant to this manuscript. Clarisse Carra-Dalliere reports no disclosure relevant to this manuscript. Mahmoud Charif reports no disclosure relevant to this manuscript. Nicolas Collongues reports no disclosure relevant to this manuscript. Jérôme de Seze reports no disclosure relevant to this manuscript. Sonia Hebbadj reports no disclosure relevant to this manuscript. Guido Ahle reports no disclosure relevant to this manuscript. Hélène Oesterlé reports no disclosure relevant to this manuscript. François Cotton reports no disclosure relevant to this manuscript. Françoise Durand-Dubief reports no disclosure relevant to this manuscript. Romain Marignier serves on scientific advisory board for MedImmune and has received funding for travel and honoraria from Biogen Idec, Merck Serono, Novartis, Sanofi-Genzyme and Teva. Frédéric Taithe reports no disclosure relevant to this manuscript. Mikael Cohen reports no disclosure relevant to this manuscript. Anne-Marie Guennoc reports no disclosure relevant to this manuscript. Anne Kerbrat reports no disclosure relevant to this manuscript. Gilles Edan reports no disclosure relevant to this manuscript. Béatrice Carsin-Nicol reports no disclosure relevant to this manuscript. Thibaut Allou reports no disclosure relevant to this manuscript. Denis Sablot reports no disclosure relevant to this manuscript. Eric Thouvenot reports no disclosure relevant to this manuscript. Aurélie Ruet or her institution received research grants and/or consulting fees from Novartis, Biogen, Merck-Serono, Bayer Healthcare, Roche, Teva, and Genzyme unrelated to the submitted work. Laurent Magy has received Honoraria, housing and consultancies from CSL Behring, Biogen, Teva Pharma, Octapharma, Pharnext, Sanofi Genzyme, Novartis. Marie-Paule Boncoeur-Martel reports no disclosure relevant to this manuscript. Pierre Labauge reports no disclosure relevant to this manuscript. Stéphane Kremer reports no disclosure relevant to this manuscript.
Ethics approval
The study was approved by Montpellier University Hospital Ethical Committee (Q-2017-03-02) and was registered on ClinicalTrial.gov (NCT03121105).
Rights and permissions
About this article
Cite this article
Ayrignac, X., Rigau, V., Lhermitte, B. et al. Pathologic and MRI analysis in acute atypical inflammatory demyelinating lesions. J Neurol 266, 1743–1755 (2019). https://doi.org/10.1007/s00415-019-09328-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09328-7